Abstract
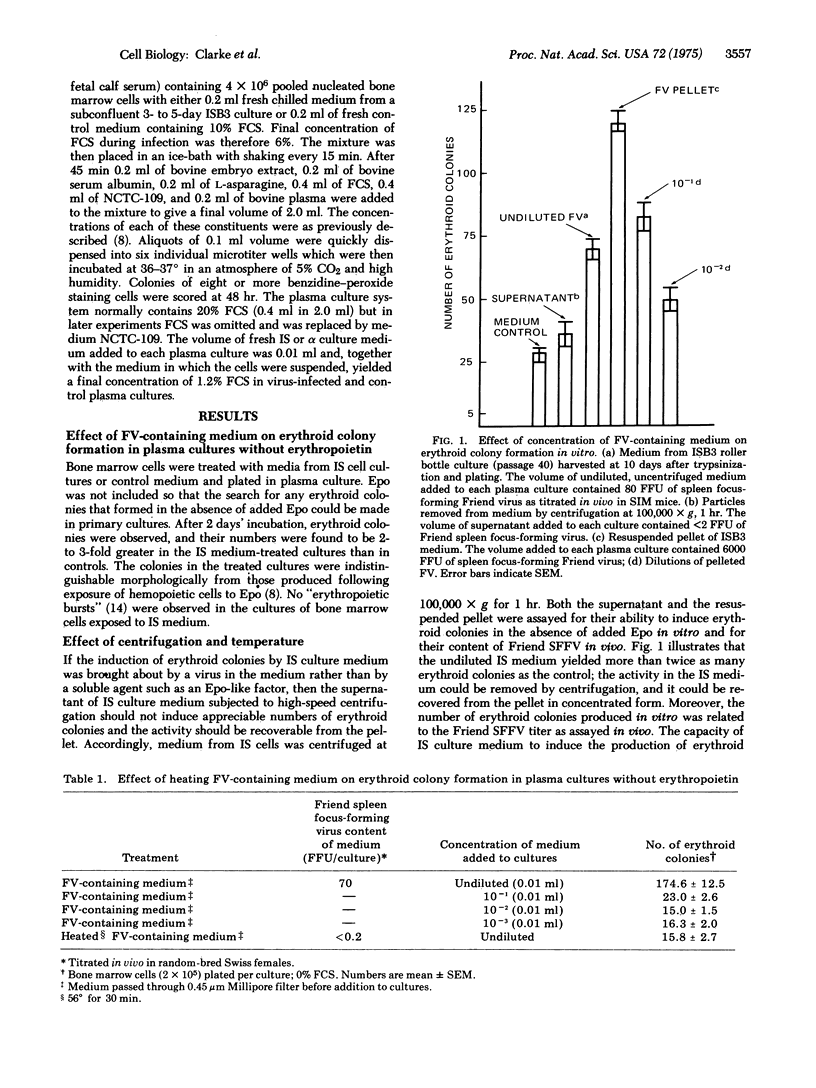
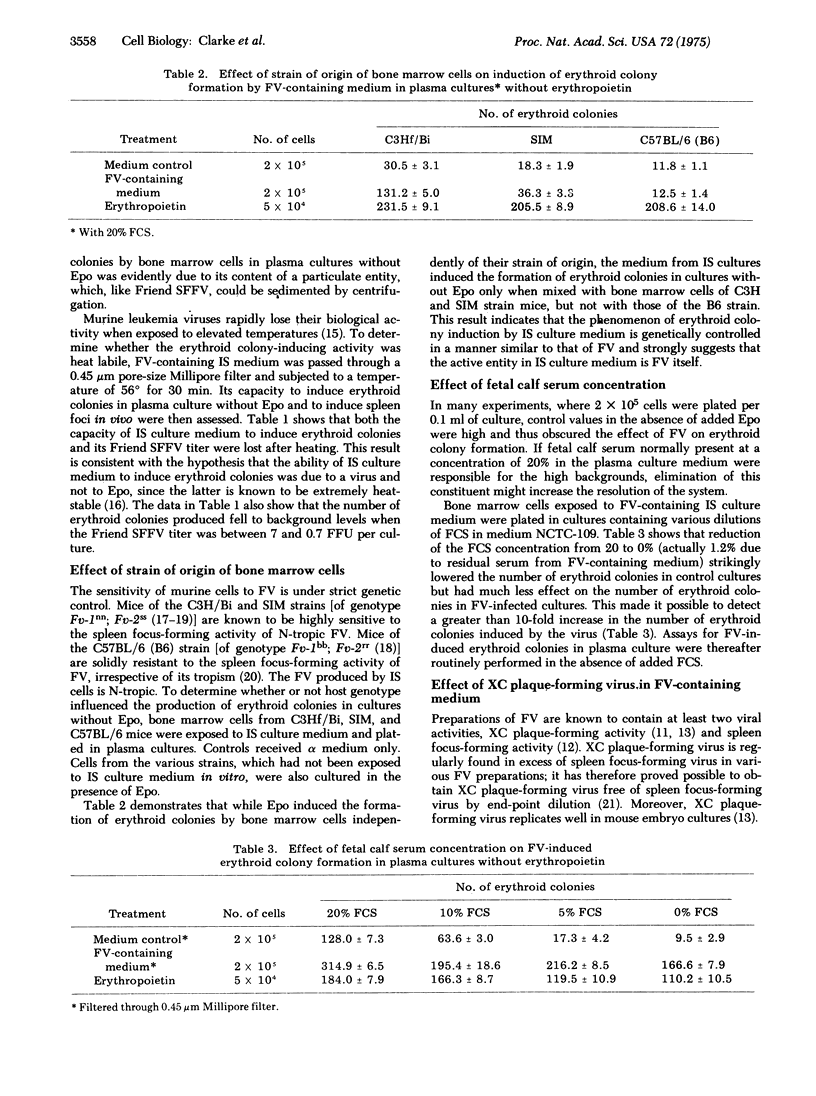
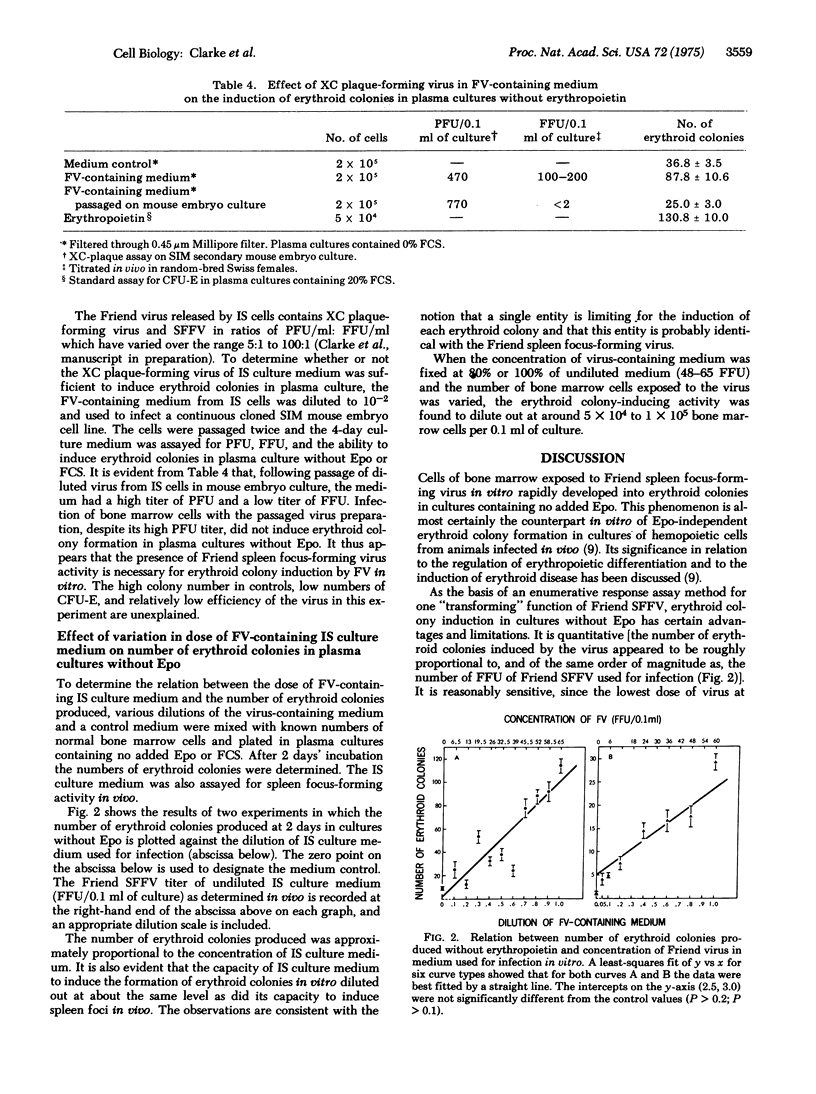
Erythroid colonies could be produced without the addition of erythropeietin in plasma cultures seeded with bone marrow cells from normal C3Hf/Bi mice by exposure of the cells in vitro to medium from a cell line (IS) that continuously produces Friend leukemia virus in culture. The activity in the culture medium was viral rather than erythropoietin-like, since it was sedimentable by high-speed centrifugation and heat labile. Erythroid colonies did not develop when the bone marrow cells exposed to virus-containing medium were from mice genetically resistant to Friend virus. IS culture medium contained both Friend spleen focus-forming and XC-plaque-forming activities. No erythroid colonies were induced when genetically sensitive cells were exposed to a preparation from which the spleen focus-forming activity had been removed, but which contained XC plaque-forming activity in high concentration. Thus the spleen focus-forming component of Friend virus appeared to be responsible for inducing erythroid colony formation without erythropoietin in vitro. Some erythroid colonies were also found in control cultures to which neither virus nor erythropoietin had been added. Reduction in the concentration of fetal calf serum in the culture medium substantially decreased the number of these colonies but had only a minor effect on the number of virus-induced colonies. The number of erythroid colonies produced after 2 days of culture without erythropoietin or fetal calf serum was approximately proportional to the titer of Friend spleen focus-forming virus to whcih the bone marrow cells had been exposed. This system should prove useful for investigation in vitro of Friend virus--host cell interactions which lead to erythropoietin-independent erythropoiesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Patuleia M. C., De Harven E. Erythrocytic maturation in vitro of murine (Friend) virus-induced leukemic cells. Natl Cancer Inst Monogr. 1966 Sep;22:505–522. [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. P., Oppenheim S., Chenaille P., Silvestre D., Tavitian A., Boiron M. Quantitative study of Rauscher virus inactivation by various physical and chemical agents. J Natl Cancer Inst. 1967 Apr;38(4):553–565. [PubMed] [Google Scholar]
- Liao S. K., Axelrad A. A. Erythropoietin-independent erythroid colony formation in vitro by hemopoietic cells of mice infected with friend virus. Int J Cancer. 1975 Mar 15;15(3):467–482. doi: 10.1002/ijc.2910150313. [DOI] [PubMed] [Google Scholar]
- METCALF D., FURTH J., BUFFETT R. F. Pathogenesis of mouse leukemia caused by Friend virus. Cancer Res. 1959 Jan;19(1):52–58. [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Mirand E. A. Murine viral-induced polycyhemia. Ann N Y Acad Sci. 1968 Mar 29;149(1):486–496. doi: 10.1111/j.1749-6632.1968.tb15187.x. [DOI] [PubMed] [Google Scholar]
- Mirand E. A., Steeves R. A., Avila L., Grace J. T., Jr Spleen focus formation by polycythemic strains of Friend leukemia virus. Proc Soc Exp Biol Med. 1968 Mar;127(3):900–904. doi: 10.3181/00379727-127-32831. [DOI] [PubMed] [Google Scholar]
- Mirand E. A., Steeves R. A., Lange R. D., Grace J. T., Jr Virus-induced polycythemia in mice: erythropoiesis without erythropoietin. Proc Soc Exp Biol Med. 1968 Jul;128(3):844–849. doi: 10.3181/00379727-128-33139. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Sassa S., Takaku F., Nakao K. Regulation of erythropoiesis in the Friend leukemia mouse. Blood. 1968 Jun;31(6):758–765. [PubMed] [Google Scholar]
- Smith H. S., Scher C. D., Todaro G. J. Induction of cell division in medium lacking serum growth factor by SV40. Virology. 1971 May;44(2):359–370. doi: 10.1016/0042-6822(71)90267-4. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J., Bennett M., Mirand E. A., Trudel P. J. Isolation and characterization of a lymphatic leukemia virus in the Friend virus complex. J Natl Cancer Inst. 1971 Jun;46(6):1209–1217. [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L. Erythroid nature of the response to Friend leukemia virus infection in mice. J Natl Cancer Inst. 1972 Feb;48(2):531–539. [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambourin P., Wendling F., Barat N., Zajdela F. Influence de différents facteurs d'homéostase érythropoïtiques sur l'évolution de la leucémie de Friend. Nouv Rev Fr Hematol. 1969 Jul-Aug;9(4):461–484. [PubMed] [Google Scholar]
- Tambourin P., Wendling F. Malignant transformation and erythroid differentiation by polycythaemia-inducing Friend virus. Nat New Biol. 1971 Dec 22;234(51):230–233. doi: 10.1038/newbio234230a0. [DOI] [PubMed] [Google Scholar]
- Thomson S., Axelrad A. A. A quantitative spleen colony assay method for tumor cells induced by Friend leukemia virus infection in mice. Cancer Res. 1968 Oct;28(10):2104–2114. [PubMed] [Google Scholar]
- Ware L. M., Axelrad A. A. Inherited resistance to N- and B-tropic murine leukemia viruses in vitro: evidence that congenic mouse strains SIM and SIM.R differ at the Fv-1 locus. Virology. 1972 Nov;50(2):339–348. doi: 10.1016/0042-6822(72)90385-6. [DOI] [PubMed] [Google Scholar]


