Figure 4.
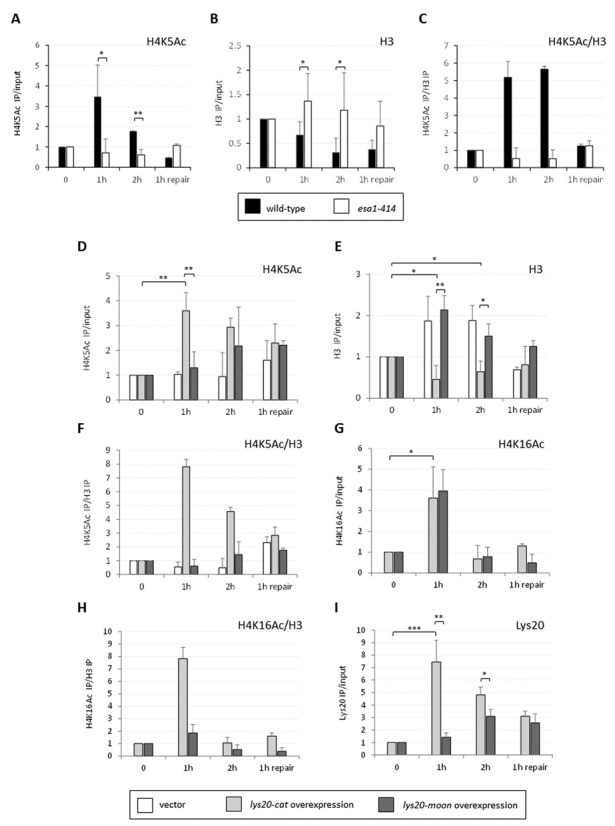
The esa1–414 cells had defective histone acetylation and eviction at the DSB. (A) Histone H4K5 acetylation was defective in esa1–414 cells compared to wild-type. ChIP anti-H4K5Ac in wild-type and esa1–414 strains. (B) Histone eviction at the break was impaired in esa1–414 cells. ChIP anti-H3Ct in strains in (A). (C) Relative H4 acetylation to histone levels increased after break induction in wild-type but not in esa1–414 cells. (D) Localized H4K5 acetylation increased when lys20-cat was overexpressed in esa1 cells 1 and 2 h after break induction. ChIP anti-H4K5Ac in esa1–414 lys20Δ lys21Δ strains transformed with vector (white), lys20-cat (light gray) or lys20-moon (dark gray). Time points and normalization are as in Figure 3E. (E) The histone eviction defect in esa1–414 cells was rescued by lys20-cat overexpression. Histone H3 levels were tested in the same strains as in (D) with anti-H3Ct ChIPs before, during and after HO break induction. (F) H4K5 acetylation relative to histone levels dramatically increased when lys20-cat was overexpressed. The enrichment of H4K5 acetylation at the break is shown relative to histone levels. Because histone H4 and H3 are heterodimers in DNA (52), comparison of histone H4 acetylation relative to histone H3 reflects histone/nucleosome occupancy. (G) Histone H4K16 acetylation increased equally in esa1 strains transformed with lys20-cat and lys20-moon. Histone H4K16 acetylation was tested in the same strains as in (D). (H) Relative histone H4K16 acetylation only increased when lys20-cat was overexpressed. H4K16 acetylation is shown relative to histone levels. (I) The moonlighting domain of Lys20 was important for recruitment to the DSBs. ChIP anti-Lys20 in a lys20Δ lys21Δ strain expressing lys20-cat or lys20-moon. Data represent three independent experiments. SD and P-values are the same as in Figure 3.
