Figure 1.
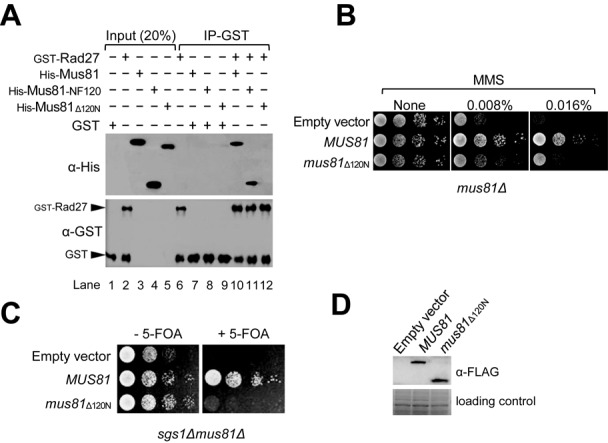
The N-terminal region of Mus81 consisting of 120 aa residues is responsible for binding Rad27. (A) Crude extracts containing GST-Rad27 (10 pmol) and Mus81–6XHis derivatives (10 pmol each; Mus81, the full-length Mus81 subunit; Mus81N120, the N-terminal 120 aa fragment; Mus81Δ120N, Mus81 lacking the N-terminal 120 aa residues) were mixed and incubated with glutathione-agarose beads for 2 h at 4°C with gentle rocking. The GST-Rad27/Mus81 complex formed was collected and washed. The presence of Mus81 derivatives and Rad27 in the precipitated materials was determined by western blotting using anti-6XHis (α-His) and anti-GST (α-GST) polyclonal antibodies, respectively. Input (20%) means the amount of proteins loaded for western blotting was 20% of total proteins used for the pull-down assays. IP-GST: GST immunoprecipitation. (B) The MMS sensitivity of mus81Δ120N was examined. The NJY1777 (sgs1Δmus81Δ + pJM500-URA3-SGS1) strain was transformed with an empty vector or vectors containing MUS81 or mus81Δ120N driven by an ADH1 promoter. The transformants were grown until saturation in liquid media and then spotted onto plates without or with indicated amounts of MMS. The plates were incubated at 30°C for 4 days. (C) The complementation of sgs1Δmus81Δ synthetic lethality by mus81 mutant alleles. The transformants (in panel (B)) were grown until saturation in liquid media and then spotted onto plates with or without 5-FOA. (D) Analysis of protein expression in 10% SDS-PAGE. The gels were stained with Coomassie blue for the loading control (bottom part). The expression of Mus81 and Mus81Δ120N was confirmed by western blotting using anti-FLAG (α-FLAG) monoclonal antibodies.
