Abstract
Eupatilin is the main active component of DA-9601, an extract from Artemisia. Recently, eupatilin was reported to have anti-inflammatory properties. We investigated the anti-arthritic effect of eupatilin in a murine arthritis model and human rheumatoid synoviocytes. DA-9601 was injected into collagen-induced arthritis (CIA) mice. Arthritis score was regularly evaluated. Mouse monocytes were differentiated into osteoclasts when eupatilin was added simultaneously. Osteoclasts were stained with tartrate-resistant acid phosphatase and then manually counted. Rheumatoid synoviocytes were stimulated with TNF-α and then treated with eupatilin, and the levels of IL-6 and IL-1β mRNA expression in synoviocytes were measured by RT-PCR. Intraperitoneal injection of DA-9601 reduced arthritis scores in CIA mice. TNF-α treatment of synoviocytes increased the expression of IL-6 and IL-1β mRNAs, which was inhibited by eupatilin. Eupatilin decreased the number of osteoclasts in a concentration dependent manner. These findings, showing that eupatilin and DA-9601 inhibited the expression of inflammatory cytokines and the differentiation of osteoclasts, suggest that eupatilin and DA-9601 is a candidate anti-inflammatory agent.
Graphical Abstract
Keywords: Eupatilin; Arthritis, Experomental; Arthritis, Rheumatoid; DA-9601
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation and bone destruction, with these processes involving various cytokines and inflammatory mediators (1). The complexity and chronicity of RA often require combined and prolonged usage of immunosuppressants, including methotrexate, leflunomide, and sulphasalazine, and biological agents such as etanercept, infliximab, and tocilizumab (2, 3). Lifelong treatment with immunosuppressive drugs is associated with significant side effects and high costs, making the proper management of RA difficult (4).
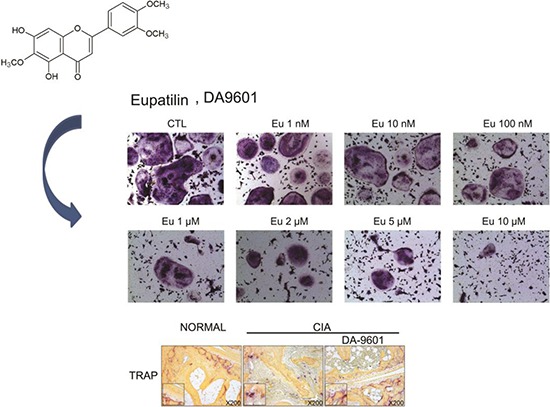
Supplementary drugs and health products with anti-inflammatory activity may benefit patients with RA. Flavonoids such as resveratrol and green tea extract are natural agents that inhibit inflammatory cytokines, osteoclast formation and Th17 differentiation in patients with RA (5, 6). DA-9601, which is extracted from Artemisia princep, is currently used clinically for mucosal protection (7, 8, 9). Eupatilin, the primary active component of DA-9601 (Fig. 1), has anti-inflammatory, anti-cancer, and anti-allergic properties, as well as a mucosal protective effect (10, 11, 12, 13). Mechanistically, eupatilin was shown to inhibit T-cell activation through intracellular calcium flux and regulation of NF-κB and NF-AT (14).
Fig. 1.
Chemical structure of eupatilin, drawn using ACD/ChemSketch free software (Advanced Chemistry Development, Inc, Tronto, Canada).
Fibroblast-like synoviocytes (FLS) have hyperplastic characteristics and a tumor-like growth phenotype, resulting in the destruction of adjacent joint structures (15). FLS plays a primary role in RA pathophysiology by producing the inflammatory cytokines tumor necrosis factor (TNF)-α and interleukins (IL)-1β and IL-6 (16). FLS also produces IL-18 and granulocyte-macrophage colony-stimulating factor (GM-CSF), which activate immune system cells (17).
Osteoclasts are often observed in RA synovium and have been associated with bone destruction. Bone-derived monocytes are easily differentiated to osteoclasts by macrophage-colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL) (18). Agents with anti-osteoclastogenic properties may therefore have a protective effect against bone destruction and osteoporosis.
This study was designed to investigate the anti-arthritic effects of DA-9601 and eupatilin in a mouse model of RA, collagen induced arthritis (CIA) mice. The anti-osteoclastogenic and anti-cytokine properties of DA-9601 and eupatilin were also analyzed in vitro using FLS from patients with RA.
MATERIALS AND METHODS
Cell culture
Synovial tissue samples removed from patients with RA were chopped finely in 20% DMEM containing 0.01% collagenase (Sigma-Aldrich, St. Louis, MO, USA) and incubated in 37℃ for 4 hr with shaking. The mixtures were centrifuged and washed once in 20% DMEM. Pellets containing FLS were cultured, with cells from passages 4 to 7 used for experiments.
Cytotoxicity assay
FLS (8,000 cells/well) were seeded in 96 well plates and incubated for 24 hr. Eupatilin was added in concentrations of 1, 10, and 100 nM and 1, 10, 50, and 100 µM, or an equal volume of dimethyl sulfoxide (DMSO). After incubation for 24 hr, 10 µL CCK-8 solution (Dojindo Molecular Technologies, Rockville, MD, USA) were added. After 2 hr, the absorbance of each well at 450 nm was determined using a microplate reader.
RNA extraction and RT PCR
FLS were incubated with 1, 2, 5, or 10 µM eupatilin or DMSO for 24 hr, followed by incubation with TNF-α (10 ng/mL) for 15 min. The cells were harvested, and total RNA was extracted with TRIZOL reagent. cDNA was synthesized using a Revertaid First-stranded cDNA Synthesis Kit (Thermo Fisher Scientific Inc, Pittsburgh, PA, USA). RT-PCR was performed using i-Taq polymerase (iNtRON Biotechnology, Seongnam, Korea) and primers for human IL-6 (sense, 5'-ATG AAC TCC TTC TCC ACA AGC GC-3'; antisense, 5'-GAA GAG CCC TCA GGC TGG ACT G-3'), IL-1β (sense, 5'-ACT GCA CGC TCC GGG ACT CA-3'; antisense, 5'-AAG GGC TGG GGA TTG GCC CT-3'; and GAPDH (sense, 5'-ACC ACA GTC CAT GCC ATC AC-3'; antisense, 5'-TCC ACC ACC CTG TTG CTG TA-3').
Osteoclast differentiation
Bone marrow was extracted from mouse limbs, and monocytes were collected. The red blood cells were lysed, and the remaining cells were washed with PBS and filtered through a strainer. The cells were centrifuged and cultured in 60 mm dishes for 1 day. Cells in suspension were counted, and 3×105 cells were incubated in α-MEM containing 10% FBS and 10 ng/mL M-CSF (PeproTech, Inc, Rocky Hill, NJ, USA) for 48 hr, followed by incubation for 72 hr with 30 ng/mL RANKL (PeproTech, Inc.) and 10 ng/mL M-CSF plus eupatilin at concentrations of 0, 1, 10, and 100 nM and 1, 2, 5, and 10 µM. After 72 hr, RANKL, M-CSF, and eupatilin were again added. The formation of multinuclear cells was observed through a microscope. Prior to becoming apoptotic, the cells were stained with tartrate-resistant acid phosphatase (TRAP) solution.
TRAP staining
After osteoclast differentiation, multinuclear cells were washed three times at 37℃ with distilled water, followed by TRAP staining (Sigma-Aldrich) for 40-60 min at 37℃. The TRAP solution was removed, and the cells were treated with distilled water. Cells with >10 nuclei were counted. For trap staining of tissue, the slide of sectioned limb tissue was deparaffinized and rehydrated. And then, the slide was washed using tap water for 5 min. TRAP solution was prepared as mixing followed solution (fast garmet GBC base solution 1,000 µL, sodium nitrite solution 1,000 µL, autoclaved water 90 mL, acetate solution 4 mL, tartrate solution 2 mL). The slide was dipped in jar with TRAP solution for 1 hr at 37℃ and incubated in jar with TRAP solution added with naphtol AS-BI phosphate solution 500 µL for 5 min. The slide was washed using tap water and stained with 2% fast green solution for 1 min.
Animals and CIA induction
Female DBA1J mice aged 6 weeks were purchased from Orient Bio, Inc. (Seongnam, Korea). Bovine type II collagen (Chondrex, Inc., Redmond, WA, USA) was dissolved in 0.05 M acetic acid overnight at 4℃. The dissolved collagen II was emulsified 1:1 in complete Freund's adjuvant (Chondrex) using a homogenizer. Each mouse was injected intradermally in the back with 100 µL emulsion and boosted by intraperitoneal injection of CII solution 21 days later. DA-9601 (100 mg/kg), dissolved in DMSO, co-solvent (PEG400:ethanol:Tween 80=85:15:5) and distilled water, was injected intraperitoneally to DA-9601 group and vehicle alone was injected intraperitoneally to CIA group and wild type group every other day. Arthritis scores in each limb were estimated on a scale of 0-4, with 0-2 indicating no, mild, and moderate redness and swelling of an ankle or paw, respectively, 3 indicating redness and swelling of an entire paw and ankle, and 4 indicating severe redness and swelling of an entire paw and ankle. Final arthritis score was calculated as an average of the four limbs.
Joint staining
Joint tissue sections were deparaffinized in xylene for 15 min and hydrated in an ethanol series. For H&E staining, the sections were incubated with Harris hematoxylin (Sigma-Aldrich) for 10 min, washed in tap water, and dipped sequentially in 1% HCl, 0.2% NH4OH, and eosin (Sigma-Aldrich) for 90 sec and washed. For safranin O staining, the sections were incubated in Weigert's iron hematoxylin (Sigma-Aldrich) for 10 min, washed in tap water for 10 min, incubated in Fast green (Sigma-Aldrich) for 5 min, dipped in 1% acetic acid 2 to 3 times, incubated in safranin O (Sigma-Aldrich) for 5 min and washed. For toluidine blue staining, the sections were incubated in toluidine blue for 3 min. Each stained section was subsequently washed in tap water, dehydrated in an ethanol series, dipped in xylene, and mounted. Inflammation and joint destruction scores were measured by three independent investigators. Inflammation scores were measured by adding scores, graded as 0-3, were based on the layer status of synovial membranes and scores, graded as 0-3, were based on the infiltration of lymphocytes. Joint destruction scores were measured by adding scores, graded as 0-3, were based on cartilage erosion, and scores, graded as 0-3, were based on pannus invasion into the cartilage. The detailed scoring method was described in reference study (19).
Flow cytometry
Mice were sacrificed, and isolated lymph nodes were stained with APC anti mouse CD4 (BD, San Jose, CA, USA). A FOXP3 stain buffer set (eBioscience, San Diego, CA, USA) was used for permeabilization and FITC anti mouse FOXP3 (eBioscience) was used for intracellular staining. Stained cells were analyzed by flow cytometry (LSR FORTESSA, BD Bioscience, San Jose, CA, USA), with the data analyzed by FlowJo 7.6.5 software (TreeStar Inc., Ashland, OR, USA).
Material
DA-9601 and eupatilin was kindly provided by Dong-A Pharmaceutical Co. Korea (Yongin, Korea).
Statistical analysis
All values are expressed as mean±SEM and compared by Student's t-tests. Statistical significance was set at P<0.05, <0.01, and <0.001.
Ethics statement
The animal studies were performed after receiving approval of the institutional animal care and use committee (IACUC) in The Catholic University of Korea (IACUC approval No. 2010-0089-05). Anonymous synovial fibroblasts were acquired from patient's tissue bank of the Catholic University of Korea, which processes were approved by Institutional review committee.
RESULTS
Eupatilin down-regulates IL-6 and IL-1β mRNA expression by TNF-α-stimulated RA-FLS
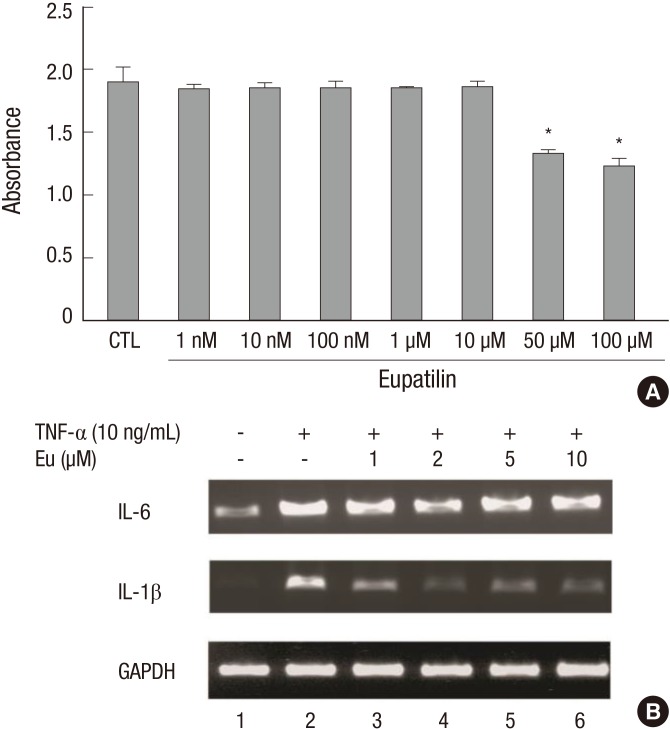
RA-FLS were treated for 24 hr with eupatilin (1, 10, and 100 nM and 1, 10, 50, and 100 µM), followed by CCK-8 for 2 hr. Eupatilin at concentrations between 1 nM and 10 µM showed no evidence of cytotoxicity (Fig. 2A). Treatment of these cells with TNF-α (10 ng/mL) for 15 min upregulated the expression of IL-6 and IL-1β mRNAs. However, pretreatment with 1-10 µM eupatilin blocked this increase (Fig. 2B).
Fig. 2.
Eupatilin suppresses mRNAs encoding inflammatory cytokines. (A) CCK assay of eupatilin cytotoxicity. FLS were seeded in 96 well plates, incubated with eupatilin for 24 hr, and incubated with CCK-8 solution for 2 hr. All values are expressed as mean±SEM. CTL (control), treatment with dimethyl sulfoxide. *P < 0.01 compared with CTL. (B) FLS were incubated with eupatilin for 24 hr and TNF-α (10 ng/mL) was added for 15 min. The amounts of IL-6 and IL-1β mRNAs were assayed by RT-PCR.
Eupatilin suppresses murine osteoclast differentiation
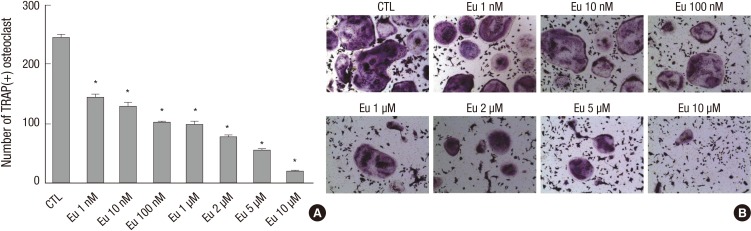
The ability of eupatilin to suppress the differentiation of murine bone marrow-derived monocytes into multi-nuclear osteoclasts (>10 nuclei) by M-CSF (10 ng/mL) and RANKL (30 ng/mL), was also tested. Whereas cells treated with DMSO yielded a mean 243 osteoclasts, treatment with 1 nM and 10 µM eupatilin yielded a mean 143 and 18 osteoclasts, respectively (P<0.001 each; Fig. 3A), showing that eupatilin reduced osteoclast formation in a concentration-dependent manner. Similarly, microscopy showed that eupatilin decreased the number of TRAP positive cells (Fig. 3B).
Fig. 3.
Eupatilin (Eu) inhibition of osteoclast formation. (A) Mouse monocytes were treated with M-CSF (10 ng/mL) and RANKL (30 ng/mL), in the presence of eupatilin or DMSO (CTL), to induce their differentiation into osteoclasts, defined as cells with >10 nuclei. Values are expressed as mean ± SEM and *P < 0.001 compared with CTL. (B) Microscopic view of the final morphology of differentiated osteoclasts. Multinuclear cells were stained with tartrate-resistant acid phosphatase (magnification, ×100).
DA-9601 attenuates experimental arthritis
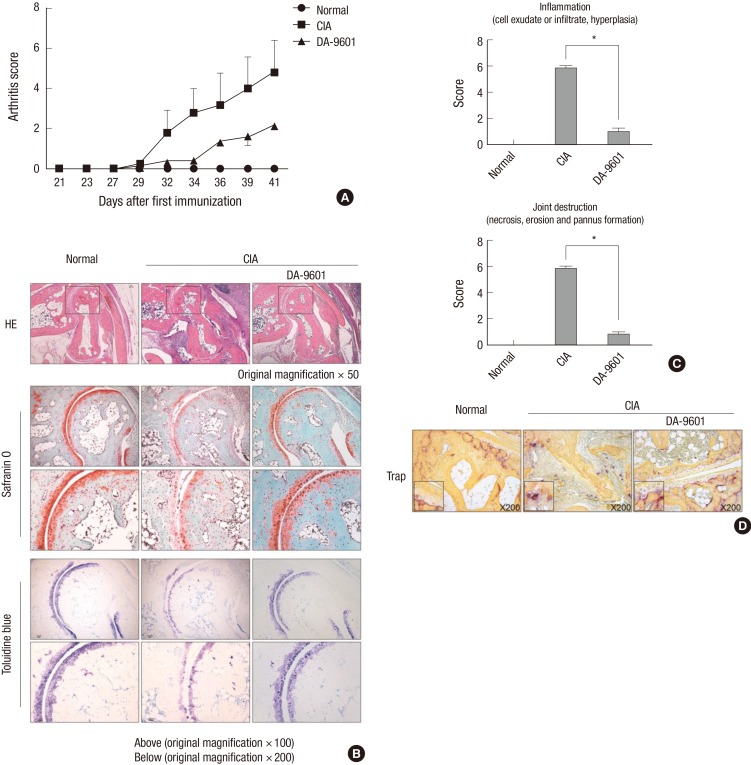
The effect of DA-9601 on experimental arthritis in vivo was assessed in a CIA mouse model. DA-9601 injection reduced the arthritis score (Fig. 4A) in these mice. Inflammation and joint destruction were reduced in mouse joints, in addition to reductions in cartilage damage and bone destruction (Fig. 4B and C). To identify that DA-9601 suppresses osteoclast caused by CIA induction in vivo, mice toe was stained with TRAP solution. The osteoclast caused by CIA induction was decreased in DA-9601-injected group (Fig. 4D).
Fig. 4.
Effect of DA-9601 on experimental arthritis. (A) Arthritis scores of mouse groups. Arthritis was induced by intradermal injection of a 1:1 emulsion of CII in CFA, followed 21 days later by an intraperitoneal injection of CII solution. The mice were injected with DA-9601 (100 mg/kg) every other day. The arthritis score represents the average degree of swelling of the four limbs. (B) Cytology of sectioned joints of mice. Hematoxylin and eosin staining shows decreased bone destruction and inflammation in the DA-9601 group compared with the CIA group. Safranin-O and toluidine blue staining show that cartilage damage by CIA was ameliorated by DA-9601. (C) Inflammation and joint destruction scores were evaluated by three investigators, as specified in the Methods section. All results are shown as mean ± SEM. *P < 0.001 for the DA-9601 compared with the CIA group. (D) The TRAP stained image of mice toe. The slide of murine toe was stained with TRAP solution. Compared with wild type, TRAP stained part of CIA group was increased. In DA-9601 group, TRAP stained part was fewer shown than CIA group (magnification, ×200).
DA-9601 increases regulatory T cell (Treg) populations in lymph nodes
To assess the effects of DA-9601 on Tregs (CD4+Foxp3+ cells), lymph nodes isolated from control, CIA, and DA-9601-treated mice were stained with appropriate antibodies and assayed by flow cytometry. Relative to the CIA group, DA-9601 increased the population of Treg cells (Fig. 5B) as well as the total number of viable Tregs (Fig. 5C).
Fig. 5.
DA-9601 increases Treg populations in lymph nodes. (A) Single cells obtained from lymph nodes were stained with anti CD4 antibody conjugated with APC and permeabilized, followed by intracellular staining with anti-Foxp3-conjugated with FITC. (B) The Treg (CD4+FOXP3+) population in the DA-9601 group was higher than in the CIA group. (C) The Treg (CD4+FOXP3+) population of CD4+ cells was higher in the DA-9601 than in the CIA group.
DISCUSSION
DA-9601 has been found to contain five active compounds, chlorogenic acid, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, jaceosidin, and eupatilin, with the latter being present in concentration at a level of 0.03±0.04 mg/g (20). Although eupatilin was shown to have anti-inflammatory properties, it had not been assessed in RA. We therefore examined the effects of eupatilin on FLS and osteoclasts, both of which are involved in the pathogenesis of RA.
FLS stimulated by TNF-α or IL-1β produces IL-6, which has both local and systemic pathogenic activity (21). At doses between 1 nM and 10 µM, eupatilin was not cytotoxic to FLS, although some cytotoxic activity was observed when FLS were treated with 50-100 µM eupatilin. At doses between 1 nM and 10 µM, eupatilin suppressed the TNF-α-induced increase in IL-6 and IL-1β mRNAs, suggesting that eupatilin may down-regulate the IL-6 and IL-1β secreted by FLS in patients with RA.
Th17 cells, which belong to the T cell lineage, secrete IL-17, which stimulates synovial fibroblasts. IL-17 and the inflammatory cytokines TNF-α, IL-1, and IL-6 stimulate synovial fibroblast cells, which can secret RANKL. RANKL induces the differentiation of precursor cells to osteoclasts, which cause bone erosion (18). We found that incubation of bone-marrow derived monocytes with eupatilin suppressed their differentiation into osteoclasts when these cells were treated with M-CSF and RANKL. These findings suggest that eupatilin, like other natural products, can inhibit factors that affect osteoclast formation (5, 22, 23, 24).
The mouse CIA model, which mimics human RA, is generated by immunization with type II collagen (CII), to which antibodies are generated in the tissue cartilage of patients with RA. This model is characterized by hyperplasia of the synovial membrane, lymphocyte infiltration, erosion of cartilage, and pannus formation. Moreover, CIA mice have specific immunity against T- and B-cells (25). By assaying limb redness swelling, we found that injection of DA-9601 reduced the arthritis score in CIA mice, indicating that DA-9601 can attenuate experimental arthritis.
Assay of lymph nodes of these mice showed that Treg (CD4+ Foxp3+) populations were higher in DA-9601-treated CIA mice than in control and CIA mice. Tregs are cells of the CD4+T cell lineage that plays a role in the induction of immune tolerance and the regulation of the immune system (26, 27). Tregs secrete the anti-inflammatory cytokine IL-10 and express Foxp3 mRNA (28, 29). Other natural agents with antioxidant activity have also been shown to up-regulates the population of Tregs (30, 31).
In conclusion, eupatilin inhibits the levels of mRNA encoding the inflammatory cytokines IL-6 and IL-1β and suppresses murine osteoclast formation. DA-9601 is a candidate to treat experimental arthritis.
ACKNOWLEDGEMENTS
The funder had no involvement in the study design, data collection, analysis, and interpretation, the writing of the manuscript, and the decision to submit the manuscript for publication.
Footnotes
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A092258).
The authors have no conflicts of interest to disclose
Conception and coordination of the study: Kim J, Ju JH, Design of ethical issues: Jung H, Jung SM, Ju JH, Acquisition of data: Kim J, Kim Y, Yi H, Jung H, Rim YA, Park N, Data review: Park SH, Ju JH, Statistical analysis: Jung SM, Manuscript preparation: Kim J, Kim Y, Ju JH, Manuscript approval: all authors.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009;5:531–541. doi: 10.1038/nrrheum.2009.182. [DOI] [PubMed] [Google Scholar]
- 3.Choy EH, Kavanaugh AF, Jones SA. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol. 2013;9:154–163. doi: 10.1038/nrrheum.2013.8. [DOI] [PubMed] [Google Scholar]
- 4.Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB. Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol. 2007;7:344–351. doi: 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Morinobu A, Biao W, Tanaka S, Horiuchi M, Jun L, Tsuji G, Sakai Y, Kurosaka M, Kumagai S. (-)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008;58:2012–2018. doi: 10.1002/art.23594. [DOI] [PubMed] [Google Scholar]
- 6.Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- 7.Choi SC, Choi EJ, Oh HM, Lee S, Lee JK, Lee MS, Shin YI, Choi SJ, Chae JR, Lee KM, et al. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappaB pathways in human gastric epithelial cells. World J Gastroenterol. 2006;12:4850–4858. doi: 10.3748/wjg.v12.i30.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seol SY, Kim MH, Ryu JS, Choi MG, Shin DW, Ahn BO. DA-9601 for erosive gastritis: results of a double-blind placebo-controlled phase III clinical trial. World J Gastroenterol. 2004;10:2379–2382. doi: 10.3748/wjg.v10.i16.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh TY, Ahn GJ, Choi SM, Ahn BO, Kim WB. Increased susceptibility of ethanol-treated gastric mucosa to naproxen and its inhibition by DA-9601, an Artemisia asiatica extract. World J Gastroenterol. 2005;11:7450–7456. doi: 10.3748/wjg.v11.i47.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park BB, Yoon J, Kim E, Choi J, Won Y, Choi J, Lee YY. Inhibitory effects of eupatilin on tumor invasion of human gastric cancer MKN-1 cells. Tumour Biol. 2013;34:875–885. doi: 10.1007/s13277-012-0621-y. [DOI] [PubMed] [Google Scholar]
- 11.Choi EJ, Oh HM, Na BR, Ramesh TP, Lee HJ, Choi CS, Choi SC, Oh TY, Choi SJ, Chae JR, et al. Eupatilin protects gastric epithelial cells from oxidative damage and down-regulates genes responsible for the cellular oxidative stress. Pharm Res. 2008;25:1355–1364. doi: 10.1007/s11095-008-9531-5. [DOI] [PubMed] [Google Scholar]
- 12.Choi EJ, Lee S, Chae JR, Lee HS, Jun CD, Kim SH. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 2011;88:1121–1126. doi: 10.1016/j.lfs.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Bae EA, Park EK, Shin YW, Baek NI, Han EJ, Chung HG, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. Int Immunopharmacol. 2007;7:1678–1684. doi: 10.1016/j.intimp.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Kim YD, Choi SC, Oh TY, Chun JS, Jun CD. Eupatilin inhibits T-cell activation by modulation of intracellular calcium flux and NF-kappaB and NF-AT activity. J Cell Biochem. 2009;108:225–236. doi: 10.1002/jcb.22244. [DOI] [PubMed] [Google Scholar]
- 15.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 19.Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, et al. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis. 2011;70:844–850. doi: 10.1136/ard.2010.132720. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Lee DY, Jeon M, Suh Y, Sung SH. Determination of five active compounds in Artemisia princeps and A. capillaris based on UPLC-DAD and discrimination of two species with multivariate analysis. Arch Pharm Res. 2014;37:617–625. doi: 10.1007/s12272-013-0204-5. [DOI] [PubMed] [Google Scholar]
- 21.Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83:585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeon JT, Kim KJ, Choi SW, Moon SH, Park YS, Ryu BJ, Oh J, Kim MS, Erkhembaatar M, Son YJ, et al. Anti-osteoclastogenic activity of praeruptorin A via inhibition of p38/Akt-c-Fos-NFATc1 signaling and PLCgamma-independent Ca2+ oscillation. PLoS One. 2014;9:e88974. doi: 10.1371/journal.pone.0088974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichikawa H, Aggarwal BB. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand and by tumor cells by suppressing nuclear factor-kappaB activation. Clin Cancer Res. 2006;12:662–668. doi: 10.1158/1078-0432.CCR-05-1749. [DOI] [PubMed] [Google Scholar]
- 24.Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. 2011;286:11492–11505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 26.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 30.Wong CP, Nguyen LP, Noh SK, Bray TM, Bruno RS, Ho E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol Lett. 2011;139:7–13. doi: 10.1016/j.imlet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad SF, Zoheir KM, Abdel-Hamied HE, Ashour AE, Bakheet SA, Attia SM, Abd-Allah AR. Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance. Int Immunopharmacol. 2013;17:79–87. doi: 10.1016/j.intimp.2013.05.026. [DOI] [PubMed] [Google Scholar]