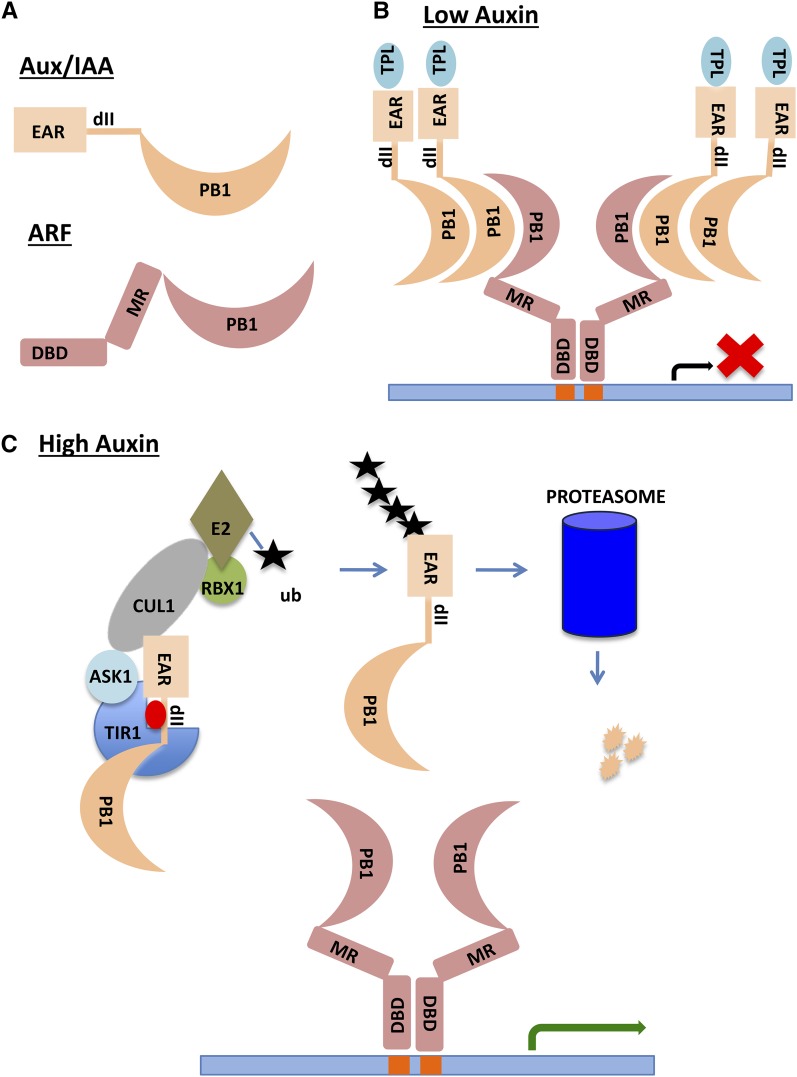
Figure 1.
SCFTIR1/AFB-Based Auxin Perception and Response.
(A) Domain structure of the Aux/IAA and ARF proteins. EAR is the ETHYLENE RESPONSE FACTOR-associated amphiphilic repression motif that interacts with the TPL corepressor. The dII domain facilitates interaction with the TIR1/AFB protein in response to auxin. The PB1 domain has both positive and negative electrostatic interfaces for directional protein interaction. DBD is the B3 DNA binding domain, and MR is the middle region that determines the activity of the ARF.
(B) Activating ARFs can form dimers through their DBDs and bind inverted repeat AuxREs (Boer et al., 2014). At low auxin levels, the Aux/IAA proteins form multimers with ARFs and recruit TPL to the chromatin. Note that most AuxREs are not found as inverted repeats in plant genomes, indicating that ARFs bind to DNA in configurations other than shown here.
(C) High levels of auxin promote ubiquitination and degradation of Aux/IAAs through SCFTIR1/AFB and the proteasome. ARFs are free to activate transcription of target genes. The site of Aux/IAA ubiquitination is arbitrary. The actual sites are unknown. Auxin is represented by the red oval.