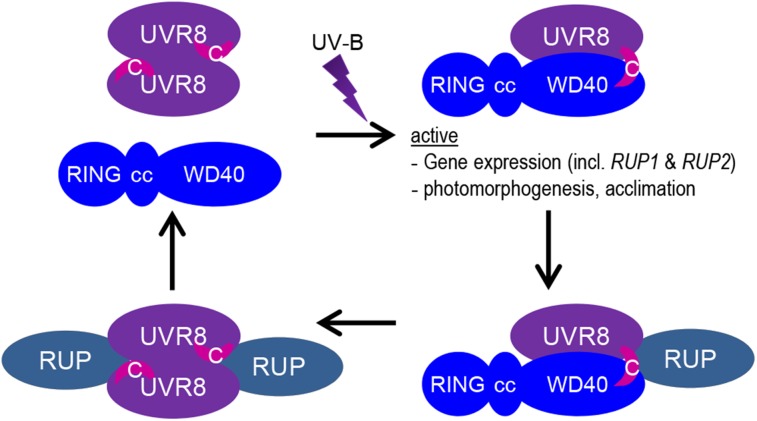
Figure 7.
Working Model of the UVR8 Photocycle.
In response to UV-B irradiation, UVR8 homodimers dissociate instantly, which allows the UVR8 seven-bladed β-propeller domain to interact with the COP1 WD40 domain (a structurally related seven-bladed β-propeller). The activated UVR8 monomer then also binds to the COP1 WD40 domain with its C27 domain (indicated by the pink crescent labeled C) and initiates UV-B signaling. Release of the C27 domain from structural constraints in the UV-B light-activated UVR8 is thought to allow its interaction with COP1. The activated UVR8-COP1 signaling pathway induces RUP1 and RUP2 expression, forming a negative feedback loop. RUP1 and RUP2 are WD40-repeat proteins that are phylogenetically and structurally related to COP1. They interact solely with the C27 domain of UVR8 and facilitate UVR8 redimerization and disruption of the UVR8-COP1 interaction. Note that no stable RUP1/RUP2-UVR8-COP1 complex is known, but it is assumed here to occur transiently when RUP1 and RUP2 attach to the C27 domain of UVR8 while UVR8 and COP1 still interact via their β-propeller surfaces. In contrast with COP1, RUP1 and RUP2 proteins are still able to interact with the C27 domain in the inactive homodimeric UVR8. The COP1-interacting SPA proteins as well as the homodimeric constitution of COP1 are omitted from the model. cc, coiled coil; RING, Really Interesting New Gene; WD40, WD40 repeat domain.