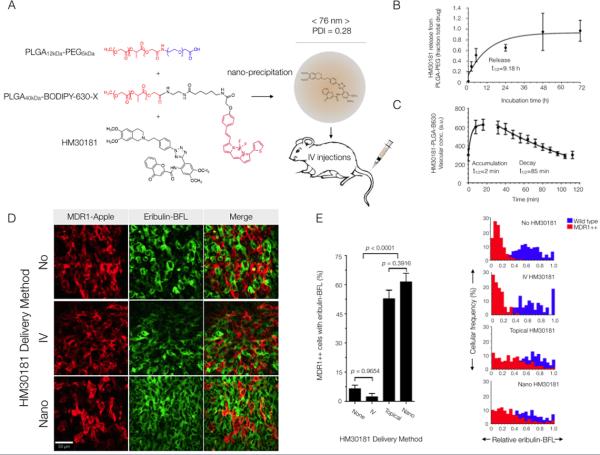
Fig. 7. Nanotherapeutic strategy for reversing MDR1-mediated drug resistance in vivo.
(A) Schematic and characterization of the PLGA-PEG-BODIPY-630 nanoparticles used to encapsulate the MDR1 inhibitor, HM30181. The particles were 76 ± 7 nm (average weighted by volume ± SD across 6 synthesis batches). (B) Release of inhibitor from nanoparticles in saline at 37°C. Data are means ± 95% confidence intervals for duplicate measurements. (C) Vascular half-life (t1/2) following rapid clearance from the tumor vasculature into surrounding tissue. Data are means +/− SEM (n = 5 vessels). (D) Eribulin-BFL accumulation in MDR1-expressing HT1080 cells in vivo without HM30181 or with daily pre-injections of HM30181, in standard solution phase vehicles or nano-encapsulated. Images are representative of 3 mice. (E) Inhibitor efficacy was a function of delivery method. The fraction of wild-type (blue) or resistant (red) cells accumulating drug was quantified for each delivery method according to the cellular frequency of eribulin-BFL concentration in MDR1-expressing cells within 1.96 SD of the mean native drug accumulation. Data are mean cellular frequencies ± SEM per imaging frame (an average of n = 300 MDR1-expressing cells evaluated per delivery method). P values were determined by one-way ANOVA using Tukey's multiple comparison test.