Abstract
The interconnection between transcription and splicing is a subject of intense study. We report that Arabidopsis homologue of spliceosome disassembly factor NTR1 is required for correct expression and splicing of DOG1, a regulator of seed dormancy. Global splicing analysis in atntr1 mutants revealed a bias for downstream 5′ and 3′ splice site selection and an enhanced rate of exon skipping. A local reduction in PolII occupancy at misspliced exons and introns in atntr1 mutants suggests that directionality in splice site selection is a manifestation of fast PolII elongation kinetics. In agreement with this model, we found AtNTR1 to bind target genes and co-localise with PolII. A minigene analysis further confirmed that strong alternative splice sites constitute an AtNTR1-dependent transcriptional roadblock. Plants deficient in PolII endonucleolytic cleavage showed opposite effects for splice site choice and PolII occupancy compared to atntr1 mutants, and inhibition of PolII elongation or endonucleolytic cleavage in atntr1 mutant resulted in partial reversal of splicing defects. We propose that AtNTR1 is part of a transcription elongation checkpoint at alternative exons in Arabidopsis.
Keywords: alternative splicing, elongation checkpoint, transcription
Introduction
Splicing is a highly complicated process that involves more than 200 proteins and five small RNAs associated with the spliceosome at different stages of splicing (Wahl et al, 2009). This enormous number of proteins is reflected in the number of molecular processes, including transcription, that are intertwined with splicing. Alternative splicing is a manifestation of this vast complexity, with more than 95% of human and 60% of Arabidopsis genes showing at least two splicing isoforms (Pan et al, 2008; Filichkin et al, 2009; Marquez et al, 2012).
One of the key plant developmental regulators with reported alternative splicing of its pre-mRNA is the DELAY OF GERMINATION 1 protein, DOG1 (Bentsink et al, 2006). The DOG1 expression level is responsible for the delay of germination in freshly harvested seeds and is regulated by various transcription elongation factors (Liu et al, 2007; Grasser et al, 2009), making it a good plant model for studying the crosstalk between splicing and elongation.
The interconnection of transcription and splicing has been extensively studied (Howe et al, 2003; Pagani et al, 2003; Chanarat et al, 2011; Close et al, 2012). Several models have been proposed to explain how chromatin regulates alternative splicing, including the direct sensing of histone modifications by spliceosome-associated factors, and influence of the transcription elongation rate on alternative splice site selection. This latter model is known as the kinetic coupling model (De la Mata et al, 2003). It is based on the observation that the changes of RNA polymerase II (PolII) elongation rate affect the selection of alternative splice sites: the slowing down of polymerase leads to exon inclusion and upstream splice site selection, while the acceleration of PolII leads to exon skipping and downstream splice site selection. Recent splicing analysis of a broad list of yeast PolII mutants, with slow and fast elongation kinetics, has confirmed the original model (Braberg et al, 2013). Although the slow PolII leads mainly to exon inclusion, there are several reports where reduced PolII elongation results in increased alternative exon skipping, including exon 9 of CFTR gene (Dutertre et al, 2010; Ip et al, 2011; Dujardin et al, 2014). For this gene, the slow elongation facilitates the binding of a negative regulator to nascent RNA that in turn results in exon skipping rather than exon inclusion (Dujardin et al, 2014).
Genetic analyses have suggested that DOG1 is a direct target of TFIIS (Mortensen & Grasser, 2014). TFIIS is an elongation factor (Sekimizu et al, 1976) required for RNA polymerase II processivity both in vitro (Izban & Luse, 1992; Reines, 1992; Cheung & Cramer, 2011) and in vivo (Sigurdsson et al, 2010). In agreement with the kinetic coupling model, tfIIs mutant shows defects in splicing of a reporter gene in yeast (Howe et al, 2003).
In addition, transient, splicing-dependent hyper-accumulation of a paused polymerase at the intron was visualised in yeast by the use of synchronised reporter system (Alexander et al, 2010). This transient transcriptional pausing event was suggested to constitute a quality checkpoint imposed by co-transcriptional splicing. This interpretation is consistent with the observed over-accumulation of PolII in humans at alternative introns and exons (Batsché et al, 2005; Saint-André et al, 2011).
NTR1 is an accessory spliceosomal component that has been characterised as an interactor of the NineTeen Complex (NTC) in yeast (Tsai, 2005; Agafonov et al, 2011). NTR1 increases PRP43 helicase activity, facilitating intron lariat release. In addition, NTR1 has been proposed to assist in the PRP43-dependent spliceosome quality checkpoint throughout the splicing cycle (Koodathingal et al, 2010; Mayas et al, 2010). In agreement with this, NTR1 has been repeatedly co-purified with the spliceosome at different stages of splicing (Cvitkovic & Jurica, 2013).
The spliceosome complex has not been purified in plants, but the Arabidopsis SPLICEOSOMAL TIMEKEEPER LOCUS 1 protein (STIPL1) has been characterised as a homologue of NTR1 (Jones et al, 2012). In agreement with this interpretation, the mutant of the Arabidopsis NTR1 homologue has extensive splicing defects and shows circadian clock defects due to the missplicing of one of the circadian clock genes (Jones et al, 2012). Surprisingly, purification of the human NTR1 complex has revealed that, in addition to its interaction with PRP43, it is co-purified with conserved group of proteins containing GCFC domain (GC-rich sequence DNA-binding factor): C2ORF3 and GCFC (Yoshimoto et al, 2014). The closest Arabidopsis homologue of C2ORF3 and GCFC is ILP1, a protein shown to bind DNA and to control endoreduplication (Yoshizumi et al, 2006).
Here, we report that the Arabidopsis thaliana NTR1 homologue (AtNTR1) is crucial for DOG1 expression and splicing. Analysis of splicing defects of DOG1 and other genes shows a strong bias towards downstream splice site selection in atntr1. In accordance with kinetic coupling model, we hypothesise that this bias is a consequence of fast PolII elongation at the splice sites in atntr1 mutant. Our PolII ChIP data revealed localised decrease in PolII occupancy at affected splice sites. This result is interpreted by us as a localised change in elongation rate. We were unable to reproduce this phenomenon using neither chemical modulation of splicing, nor mutants in other splicing factors, which proves that localised decrease in PolII occupancy is AtNTR1-specific. This interpretation is consistent with observed immuno-co-localisation of AtNTR1 with PolII in the nucleus and the presence of AtNTR1 at DNA of its target genes as shown by ChIP. Analysis of AtNTR1-dependent splicing events showed that NTR1 is required for splicing of strong, consensus-like, alternative splice sites. This was corroborated by mutational analysis that showed an atntr1-dependent increased accumulation of PolII ChIP signal at the strong alternative splice sites. Our data are consistent with NTR1 being required for co-transcriptional pausing of polymerase at strong alternative splice sites. We therefore interpret the directionality of alternative splicing defects in atntr1 mutant as a manifestation of PolII elongation defects.
The role of transcription elongation in alternative splice site selection has been extensively studied (De la Mata et al, 2011). To investigate whether alternative splice site selection in plants also depends on transcription elongation rate, we have compromised PolII elongation by mutating TFIIS and exposed plants to 6AU (6-azauracil) and MPA (mycophenolic acid) treatment. Observed changes in alternative splicing were predominantly opposite to ones observed in atntr1 mutant and consistent with prediction based on the kinetic coupling model, supporting our conclusions.
Results
AtNTR1 regulates seed dormancy, DOG1 expression and splicing
The NTR1 homologue in Arabidopsis was originally identified by means of genetic screen aimed to identify circadian clock regulators (Jones et al, 2012). We found that in addition to its role in circadian clock regulation, atntr1-1 mutants showed pleiotropic phenotypes including low seed dormancy, altered flowering time, altered leaf morphology and enhanced lethality at elevated temperatures (Fig1A, Supplementary Fig S1). We focused on the seed dormancy phenotype and confirmed that both the available alleles, atntr1-1 and atntr1-2, showed enhanced germination without stratification (Fig1A and B, Supplementary Fig S1A). Interestingly, dog1 mutants have been shown to have similar phenotype (Bentsink et al, 2006). In agreement with the low seed dormancy phenotype, we found reduced expression of DOG1 gene in atntr1 mutants, both in seeds (Fig1C) and seedlings (Supplementary Fig S1B). Because AtNTR1 deficiency leads to massive splicing defects in Arabidopsis (Jones et al, 2012) and DOG1 is a subject of alternative splicing (Bentsink et al, 2006), we analysed the splicing defects of the DOG1 transcripts. The atntr1-1 mutation resulted in more pronounced usage of the 5′ downstream splice site, with a concomitant reduction in the upstream 5′SS selection in comparison with wild-type plants (Fig1D). Additionally, an approximately 50% increase in intron retention was observed (Fig1D). The altered splicing isoforms corresponded to the most abundant splice isoforms of DOG1, namely alpha and beta (Bentsink et al, 2006; Schwab, 2008). Consequently, we have measured all four isoforms reported for DOG1 and found that, indeed, isoforms alpha and beta were the most affected (Supplementary Fig S1C and D).
Figure 1.
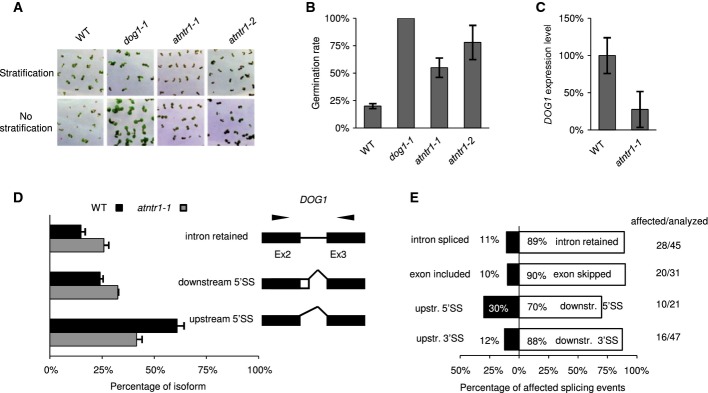
- Photographs (A) and quantification (B) of seed dormancy tests. The chart represents the average percentage of germinated seeds without stratification after 4 days of growth in LD. The error bars represent ± SE (n = 3). Tests were performed on freshly harvested seeds, with or without 3 days of stratification growth in LD.
- qPCR of DOG1 expression in siliques (16 days after pollination). The graph represents the average ratio of DOG1 to UBC, normalised to Col-0 (WT). The error bars represent ± SE (n = 3).
- DOG1 splicing was assessed by RT–PCR combined with capillary electrophoresis. The graph represents the mean relative contribution of the mRNA forms found in the total pool of amplified products. The black and grey bars represent the data for Col-0 and atntr1-1, respectively. The error bars represent ± SD (n = 3). To the right of the charts, the structures of the examined transcripts are shown (black boxes, constitutive exons; white boxes, alternative regions; black lines, introns). The black arrows show the locations of primers. Downstr. and upstr. stand for downstream and upstream splicing event, respectively.
- Directionality of splice site selection in atntr1. Splicing was analysed in 14-day-old MS grown plants. For each type of alternative splice event, the black and white boxes show the contributions of opposite direction splicing events. The numbers represent the percentage of splice events supporting the direction of the splice site event change (also shown on horizontal axis). The numbers on the right-hand panel represent the number of affected splicing events versus total number of splicing events analysed. The white bars represent distal 3′ and downstream 5′ splice site selection (3′SS/5′SS), exon skipping (ES) and intron retention (IR), while the black bars represent 5′ and 3′ splice site selection (3′SS/5′SS), exon inclusion (ES) and intron splicing (IR).
The atntr1 mutant shows bias in alternative 5′ and 3′ splice site selection
We were intrigued by the change in splice site selection on DOG1 towards the downstream splice site. DOG1 expression strongly depends on factors required for efficient transcription elongation (including TFIIS). The observed tendency towards downstream splice site selection in the NTR1 mutant could be a manifestation of a defect in the PolII elongation rate (Liu et al, 2007; Mortensen & Grasser, 2014). We therefore extended our observation of alternative splicing changes. Independently of the previous report, a selection of 144 alternative splice events were analysed in atntr1-1 and in wild-type plants (Jones et al, 2012). In agreement with previous results, we found that the most abundant splicing defects were intron retention and exon skipping (Fig1E). For 144 alternative splicing events analysed, 74 were significantly changed. Prominent bias in the directionality of alternative 5′ splice site selection was observed, which is in accordance with the directionality of splice site selection on DOG1 (Fig1D and E). Of 16 affected alternative 3′ splice site selection events, 14 (88%) were changed in atntr1-1 towards downstream splice sites (SS). Similar bias was also observed in the directionality of splicing events in the case of 5′ splice site events (seven of 10 changed towards downstream SS—70%) and in exon skipping events (18 of 20 changed towards exon skipping—90%) (Fig1E and Supplementary Table S1).
Upstream/downstream splice site selection has been proposed to represent a manifestation of the polymerase II elongation rate (De la Mata et al, 2003, 2011). Consequently, the observed bias could indicate a defect in transcription elongation across the affected splice sites in the atntr1-1 mutant.
AtNTR1 is required for splicing of strong consensus splice sites
Next, we wanted to understand what creates specificity for AtNTR1 at some splice sites but not the others. Analysis of acceptor splice sites sequences showed no clear difference between AtNTR1-dependent and AtNTR1-independent introns (Supplementary Fig S2B). On the other hand, analysis of donor sites revealed a significant difference at position +3/+4 (P-value < 0.05, Fisher's exact test). AtNTR1-dependent introns show a higher likelihood of A/G at +3 and A at +4 positions compared to AtNTR1-independent splice sites (Supplementary Fig S2A). The consensus donor splicing site sequence in Arabidopsis is AG|GTAAGT. Consequently, the affected introns more closely resemble the whole-genome consensus than introns with splicing unaffected in atntr1-1.
We decided to test whether the AtNTR1 requirement for splicing is specified by the strong sequence of alternative splicing donor splice site as has been suggested by our sequence analysis. We selected an alternative 5′SS event that is not dependent on NTR1 and has week consensus sequences at the upstream and downstream splicing sites. Subsequently, those sites were mutated into strong consensus sequences by changing two nucleotides at each site (Supplementary Fig S1C). Analysis showed that whereas splicing of the native version (5′SS wt) was not changed in atntr1 mutant, the splicing of the mutated construct (5′SS strong) was affected (Supplementary Fig S1D). Although the change in alternative splice site selection was modest, it was statistically significant (t-test P-value < 0.01). This confirms our initial observation that the 5′SS consensus with extended homology to U1 constitutes a preferable target for NTR1 splicing activity. In addition, this analysis shows that the AtNTR1 effect on splicing is downstream of splice site recognition by the spliceosome, which is consistent with the AtNTR1 role in recycling of U6.
In addition to U6/5/2 snRNPs, AtNTR1 interacts with U1
To obtain more insights into the potential function of AtNTR1, RNA molecules associated with AtNTR1 were analysed using RNA immunoprecipitation (RIP) followed by RT–PCR. In this experiment, a complementing transgenic Arabidopsis line expressing the AtNTR1-GFP fusion protein in the atntr1-1 genetic background was used with antibodies recognising GFP. Given the well-documented role of NTR1 in U6, U5 and U4 recycling, we tested AtNTR1 interaction with those molecules. We could clearly observe an enrichment of U6, U5 and U2 RNA in the AtNTR1-GFP-immunoprecipitated fraction, compared to our negative control (Fig2A). Surprisingly, a strong and reproducible interaction of AtNTR1 with U1 was also observed (Fig2A). In contrast, U3 and 18S rRNA showed no enrichment, confirming the stringency of our method (Fig2A). This result was further confirmed by the identification of AtNTR1-associated proteins by means of mass spectrometry. The U1 associated protein—U1A—was one of our highest-scoring interactors (Table1).
Figure 2.
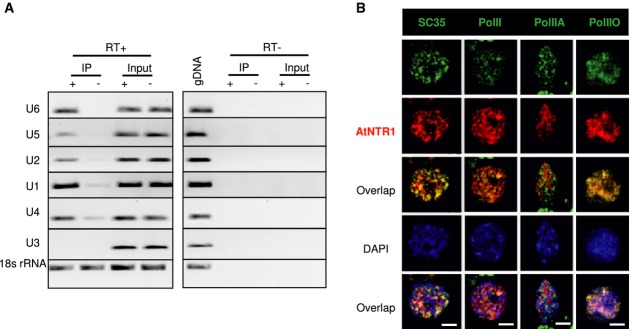
- Electrophoresis of RT–PCR products showing interactions of AtNTR1 with selected snRNA targets detected by RIP. The level of transcripts co-precipitated from transgenic plants expressing ANTR1-GFP (IP+) or wild-type plants (IP−) using anti-GFP antibody was measured by RT–PCR normalised to the inputs. To control the amplification from gDNA, controls without reverse transcriptase (RT) were performed. U3 snRNA and 18S rRNA were used as negative controls for interaction.
- Fluorescent immunostaining of nuclei showing the co-localisation of AtNTR1 with SC35, total PolII, Ser5-phosphorylated PolII (PolIIA) or Ser2-phosphorylated PolII (PolIIO). AtNTR1 was detected using an antibody raised against AtNTR1 peptide. Scale bar represents 2.5 μm.
Table 1.
AtNTR1 co-purifying proteins.
| Gene ID | Gene name | MW (Da) | Number of unique peptides P1-P2-P3-P4 |
|---|---|---|---|
| AT1G17070 | AtNTR1 | 96,937 | 37-36-26-30 |
| AT5G08550 | ILP1 | 100,998 | 21-22-30-28 |
| AT1G24180 | IAR4 | 43,787 | 1-2-2-2 |
| AT2G39770 | CYT1 | 39,837 | 1-1-1-2 |
| AT2G47580 | U1A | 58,456 | 1-1-1-1 |
| AT2G30050 | WD40 | 32,907 | 1-1-1-1 |
Seedlings of complementing AtNTR1-GFP transgenic lines, expressed under native promoter, were used for four independent purifications with three negative controls (Col-0). After trypsin digestion and mass spectrometry, proteins identified in all purifications but not in negative controls were listed in the table. Number of unique peptides matching each identified protein is shown separately for each purification (P1–4).
ILP1, a GCFC domain-containing protein, interacts with AtNTR1 and is required for efficient splicing
The highest-ranking NTR1 interactor on our list was a protein known as ILP1 in Arabidopsis (Yoshizumi et al, 2006). ILP1 contains the GCFC domain (GC-rich sequence DNA-binding factor-like domain) and is a homologue of the human proteins C2ORF3 and GCFC (also known as Pax3/7BP) (Diao et al, 2012; Yoshimoto et al, 2014). Both, the bimolecular fluorescence complementation (BiFC) assay using YFP (Supplementary Fig S3B) and an yeast two-hybrid assay (Supplementary Fig S3C), confirmed our original finding and suggested direct AtNTR1–ILP1 interaction. Interestingly, homologues of ILP1 in human co-purify with TFIP11, a human homologue of AtNTR1 (Yoshimoto et al, 2014). This indicates that this interaction is conserved between species, which suggests that it may be important for NTR1 function. ILP1 in Arabidopsis binds to a promoter of a key cell cycle gene and controls its expression, providing a possible explanation for endoreduplication defects in the ilp1 mutant (Yoshizumi et al, 2006). In addition, human homologues of ILP1 likewise bind to gene promoters to regulate their expression (Diao et al, 2012).
Next, we tested whether ILP1 was involved in splicing regulation in Arabidopsis. The atntr1-1 and ilp1-1 mutants' analysis revealed that ilp1-1 had very strong splicing defects, with virtually all splicing events affected in atntr1-1 also being misregulated in the ilp1 mutant (Supplementary Fig S3D, Supplementary Table S2). This finding is consistent with the recent data on a human ILP1 homologue showing that the depletion of C2ORF3 by RNAi repressed pre-mRNA splicing in vitro (Yoshimoto et al, 2014).
We conclude that ILP1, like its human homologues, is a direct interactor of AtNTR1 and that GCFC domain-containing proteins are required for efficient splicing both in Arabidopsis and in humans.
PolII co-localises with AtNTR1
AtNTR1 immunolocalisation was investigated, to test the relationship between AtNTR1 and PolII. We confirmed previous results from human cells showing that NTR1 is localised in the nucleus but excluded from the nucleolus, using a complementing genomic NTR1-GFP line (Supplementary Fig S1I and J) and AtNTR1 antibody (Fig2B) (Tannukit et al, 2008). In addition, it was found that AtNTR1 is only partially co-localised with the SC35 splicing factor using dual labelling (Fig2B), which is in agreement with results concerning NTR1 mouse homologue (Wen et al, 2005). Subsequently, we investigated the co-localisation of AtNTR1 with PolII. Three different PolII antibodies were used: the first recognising all forms of PolII (total PolII), the second recognising the Ser5-phosphorylated form of PolII (PolIIA) that is usually associated with the initiation of transcription, and the third recognising Ser2-phosphorylated PolII (PolIIO), which is believed to primarily mark PolII associated with gene bodies. Partial co-localisation with total PolII was observed. It could be attributed to the Ser2-phosphorylated form of PolII, based on observed no co-localisation with PolIIA and strong co-localisation of AtNTR1 with the PolIIO (Fig2B). Although the functional distinction between Ser5-phosphorylated and Ser2-phosphorylated PolII is not absolute, it is generally believed that Ser2 phosphorylation is a mark of elongating polymerase (Komarnitsky, 2000; Buratowski, 2009).
AtNTR1 acts co-transcriptionally at affected splice sites
The co-localisation of AtNTR1 with Ser2-phosphorylated PolII suggests that NTR1 can be physically present at the target genes. In order to test this, AtNTR1 localisation on the DOG1 gene was analysed by chromatin immunoprecipitation (ChIP) using antibodies that recognise AtNTR1.
Our ChIP analysis shows that AtNTR1 is present at the gene body and promoter of DOG1, in contrast to an intergenic region selected as a negative control (Fig3). In addition, a set of five other genes was tested for NTR1 presence. Clear NTR1 signal could be detected on all of them (Supplementary Fig S4). The genes were selected from set that displayed misregulated alternative splicing in atntr1. Moreover, the five genes were chosen to represent different types of alternative splicing events. The physical presence of AtNTR1 on those genes substantiates our NTR1 PolII co-localisation data and suggests that at least some of NTR1 activity is happening co-transcriptionally.
Figure 3.
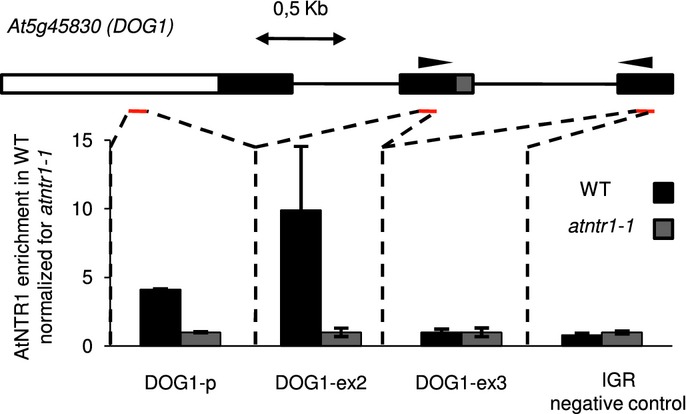
AtNTR1 is present at the DOG1 gene
AtNTR1 antibodies were used to analyse AtNTR1 protein presence at DOG1 locus using ChIP. Data shown represent enrichment above background level measured in atntr1-1 mutant. Gene structure is shown with black boxes representing constitutive exons; grey box, alternative region; white box, promoter region; black lines, introns. Red lines show amplified regions. 0.5 kb scale is shown. Error bars represent ± SD of three independent experiments. As an additional negative control, primers amplifying an unlinked intergenic region (IGR) were used.
We conclude that AtNTR1 is present at or close to DNA of genes that display AtNTR1-dependent splicing. In addition, our ChIP analysis shows that AtNTR1 is present throughout the analysed genes, with no clear enrichment at misspliced introns. This result is consistent with immuno-co-localisation studies of AtNTR1 and PolII.
Localised PolII level reduction in atntr1 mutant
Our data show a strong link between AtNTR1 and PolII. Moreover, the splicing defects observed in the NTR1 mutant could be a manifestation of fast PolII elongation across these splice sites. We therefore considered a possibility that AtNTR1 is involved in the control of PolII at those splice sites. To address this, the genes that previously showed to be direct AtNTR1 targets were used to assess PolII profile by ChIP using antibodies that recognise total PolII.
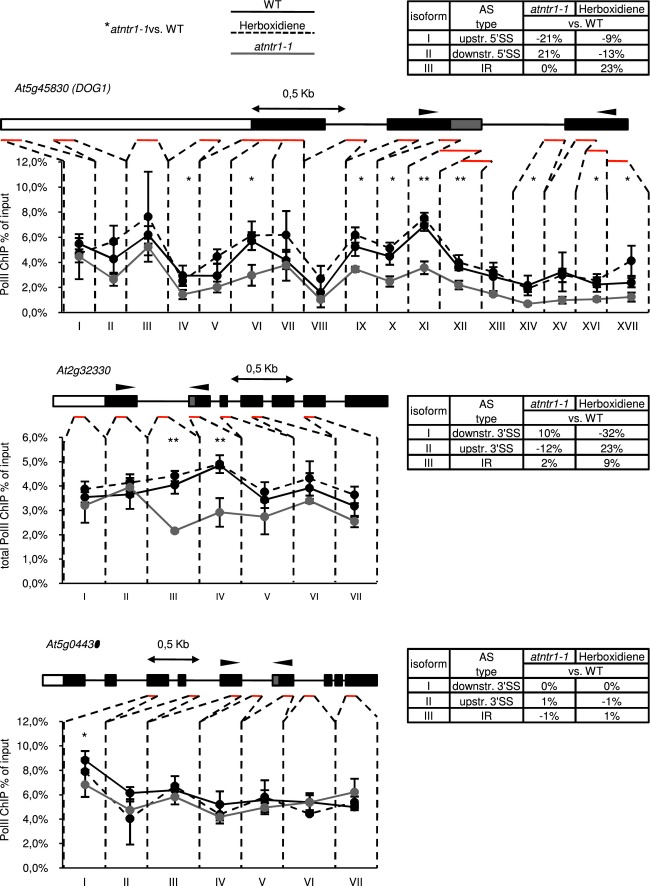
Analysis of PolII occupancy in atntr1-1 consistently revealed a significant reduction in PolII for all genes with AtNTR1-dependent splicing (five of the five genes tested) (Fig4 and Supplementary Fig S5). With an exception of DOG1, the reduction was limited to or strongest at the regions of misspliced introns. The At5g04430 gene was analysed as a control, as it shows atntr1-independent alternative splicing and consequently showed no significant reduction in PolII levels in atntr1 mutant.
Figure 4.
atntr1, in contrast to herboxidiene-treated plants, shows localised decrease in PolII occupancy on alternatively spliced exons
Line charts present ChIP profile of total PolII on examined genes. Black, grey and dashed black lines represent results for Col-0, atntr1-1 and herboxidiene-treated WT plants, respectively. Above each chart, gene structure is shown with black boxes representing constitutive exons; grey boxes, alternative regions; white boxes, promoter region; black lines, introns. Red lines show amplified regions. Above each gene structure, 0.5 kb scale is shown. For each chart, the mean value from three independent experiments is shown. Error bars represent ± SD, **P < 0.01 and *P < 0.05 of t-test. Arrows on gene structure show localisation of primers used for splicing analysis by RT-PCR, which was followed by capillary electrophoresis (results shown in tables next to each gene). Tables represent splicing site selection in mutant and herboxidiene-treated plants in comparison with wild-type.
Source data are available online for this figure
Reduction in PolII levels in atntr1 is not a consequence of reduced splicing efficiency
Research in yeasts suggests that aberrant splicing may lead to stronger PolII pausing (Alexander et al, 2010; Chathoth et al, 2014). In the case of atntr1, we observe the reduction in PolII accumulation across the intron associated with aberrant splicing. It is possible that the reduction in PolII levels observed in atntr1 could be a consequence of general splicing malfunction in this mutant. To validate this possibility, we tested whether PolII occupancy defects observed in atntr1 could be mimicked by alteration of splicing. We first used the chemical modulator of splicing herboxidiene. Herboxidiene is a plant herbicide, which was shown to modulate splicing in humans by direct binding to the spliceosome component SAP155 (Miller-Wideman et al, 1992; Hasegawa et al, 2011; Lagisetti et al, 2014).
Our results show clear alteration of splicing across nearly all analysed splicing events in the case of WT plants treated with herboxidiene (Fig4, Supplementary Fig S5 and Supplementary Table S3). However, the PolII ChIP occupancy analysis in herboxidiene-treated plants failed to show any significant reduction in PolII levels at the affected splice sites (Fig4, Supplementary Fig S5). Both herboxidiene and spliceostatin A target the same splicing factor (Kaida et al, 2007). That leads to the conclusion that our data are in agreement with data from human studies where spliceostatin A treatment did not cause change in PolII levels or elongation kinetics at the splice sites (Brody et al, 2011). Taken together, our results suggest that the reduction in PolII levels in atntr1 is not a consequence of misregulated splicing but rather a direct affect of NTR1 deficiency.
In addition to chemical modulation of splicing, we tested two splicing mutants known to regulate alternative splicing, namely sr45-1 and smd3-b (Palusa et al, 2007; Swaraz et al, 2011). Our analysis showed no change in PolII occupancy on any of the six tested genes (Supplementary Fig S6). In contrast, we have found a substantial change in splicing of those genes. These changes were often similar to ones observed for the atntr1 mutant (Supplementary Fig S6 and Supplementary Table S4). This shows that the PolII occupancy change observed in atntr1 is not shared among all splicing factor mutants but is specific for the NTR1.
The reduction in PolII level in atntr1 is unlikely to represent a reduction in overall transcription rate due to the fact that, in majority of cases, it is localised in the vicinity of affected splice sites and cannot be observed throughout the whole gene.
The observed localised decrease in PolII occupancy in atntr1 mutant could be interpreted in several ways. Given the directionality in splice site selection, we interpret the altered PolII profile as a result of localised change of elongation rate across the affected splice sites. The PolII occupancy analysis in the atntr1 mutant supports a model, in which the NTR1 facilitates the splice-site-dependent pausing at alternative splice sites in Arabidopsis. Alternative splice sites have been shown to accumulate high PolII signal in humans (Saint-André et al, 2011). We however could not detect a significant increase in the PolII signal at alternative splice sites when compared to surrounding regions in WT plants, suggesting that there are many parallel mechanisms controlling PolII occupancy at those genes.
AtNTR1 induces pausing of PolII at strong alternative splices sites on the transgene
We previously showed that splicing of our minigene became NTR1 dependent when its sequence was mutated into strong splice site consensus. Next, we wanted to investigate the PolII occupancy using minigenes. To do this, we first analysed transgenic lines with WT alternative 5′ donor splice sites (5′SS wt) and mutated NTR1-dependent strong upstream—strong downstream splice sites (5′SS strong). The 5′SS strong transgene compared to 5′SS wt transgene showed significantly higher PolII levels, specifically at the studied splice site (Fig5). The 5′SS strong construct differs from the 5′SS wt construct only by 2 nucleotides mutated at upstream and downstream 5′SS, respectively. The dependence of the localised increase in PolII occupancy in our minigene suggests a PolII pause site, controlled by the spliceosome, operates at strong alternative splice sites on our transgene. Localised increase in PolII occupancy is dependent on the presence of strong alternative splice sites. This shows that, in the context of the transgene, the PolII pause site requires strong consensus sequence and suggests PolII pausing is controlled by the spliceosome.
Figure 5.
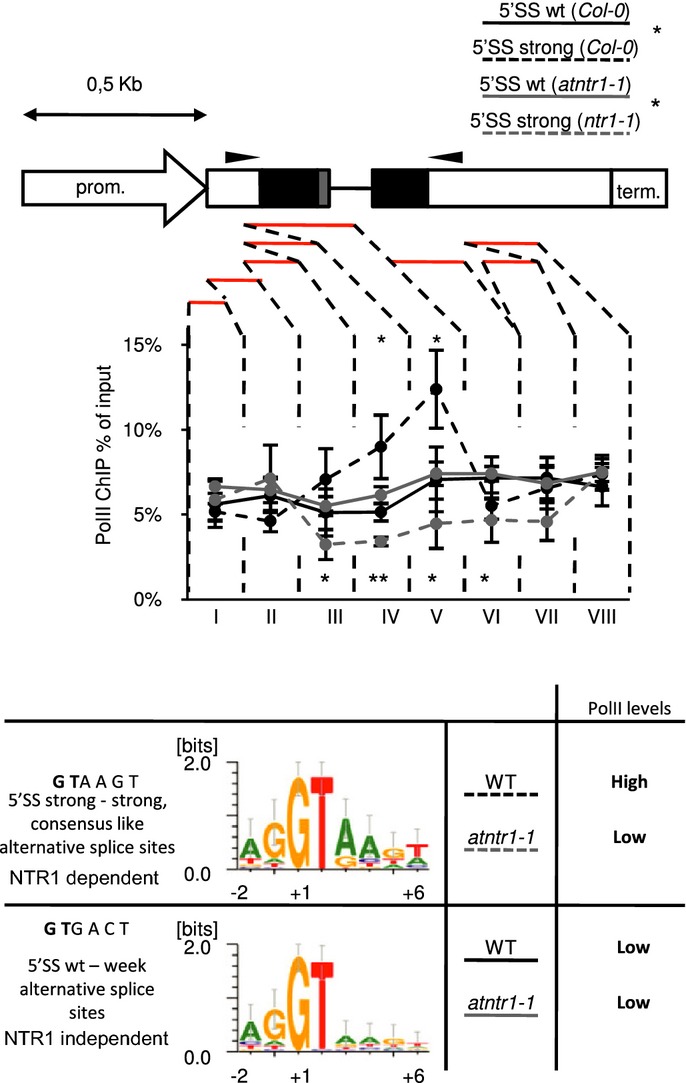
Strong alternative splice sites constitute an AtNTR1-dependent PolII pause site on the minigene
Transgenic plants expressing 5′SS wt and 5′SS strong constructs were used to perform ChIP experiments using total PolII antibodies. Line charts present ChIP profile of total PolII on minigene. Reporter gene structure is shown with black boxes representing constitutive exons; grey boxes, alternative region; white boxes, promoter and terminator regions; black lines, introns. Red lines show amplified regions. Above gene structure, a 0.5 kb scale is shown. Arrows on gene structure show localisation of primers used for splicing analysis. Error bars represent ± SD of three independent transgenic lines, **P < 0.01 and *P < 0.05 of t-test. Bottom panel shows a schematic representation of 5′SS strong-NTR1-dependent and 5′SS wt-NTR1-independent consensus as described in Materials and Methods.
Source data are available online for this figure
Next we tested whether this PolII pausing on our minigene is NTR1 dependent. Analysis performed in the atntr1-1 background showed a marked reduction in PolII levels for 5′SS strong but not for the 5′SS wt construct (Fig5). Those results confirmed that AtNTR1 is required for high PolII occupancy observed on the transgene. We interpret this localised PolII occupancy change as an AtNTR1-dependent transcriptional pausing at strong alternative splice sites of the transgene.
Analysis of endogenous targets in WT plants revealed no substantial increase in PolII occupancy at alternative splice sites compared to surrounding regions. Therefore, we can only speculate that on endogenous genes, similarly to our transgene strong alternative splice sites constitutes a transcriptional pause site, but other PolII elongation control mechanisms mask the result. This interpretation is consistent with the observed reduction in PolII occupancy level observed in atntr1 mutant at endogenous targets.
The locally increased PolII occupancy, observed at 5′SS strong compared to 5′SS wt construct, together with localised reduction in PolII in atntr1 both at transgene and endogenous targets, suggests a role of AtNTR1 in transient transcriptional pausing at splice sites. Using chemical or genetic interference, we could not reproduce PolII decrease observed in atntr1 mutant. Therefore, we interpret the PolII changes detected in atntr1 not as a consequence of aberrant splicing but rather as a manifestation of AtNTR1 function.
The dominant-negative TFIIS mutant blocking PolII endonucleolytic cleavage shows upstream splice site selection
The directionality of alternative splice site selection and the localised PolII occupancy decrease in atntr1 are interpreted by us as a consequence of fast PolII elongation in this mutant. It was therefore tested whether in Arabidopsis, as in other systems, the reduction in PolII elongation will result in upstream splice site selection and exon inclusion (De la Mata et al, 2003; Ip et al, 2011). Given that DOG1, one of our target genes, has been shown to be regulated directly by TFIIS, we focussed our attention on this mutant (Mortensen & Grasser, 2014). TFIIS is a conserved component of the PolII holoenzyme, required for efficient PolII endonucleolytic cleavage activity (Sigurdsson et al, 2010). TFIIS has a modulatory function in the enhancement of PolII processivity, allowing reinitiation of backtracked, paused polymerase. TFIIS mutants have been shown to display splicing defects in yeast and humans (Howe et al, 2003; Shukla et al, 2011).
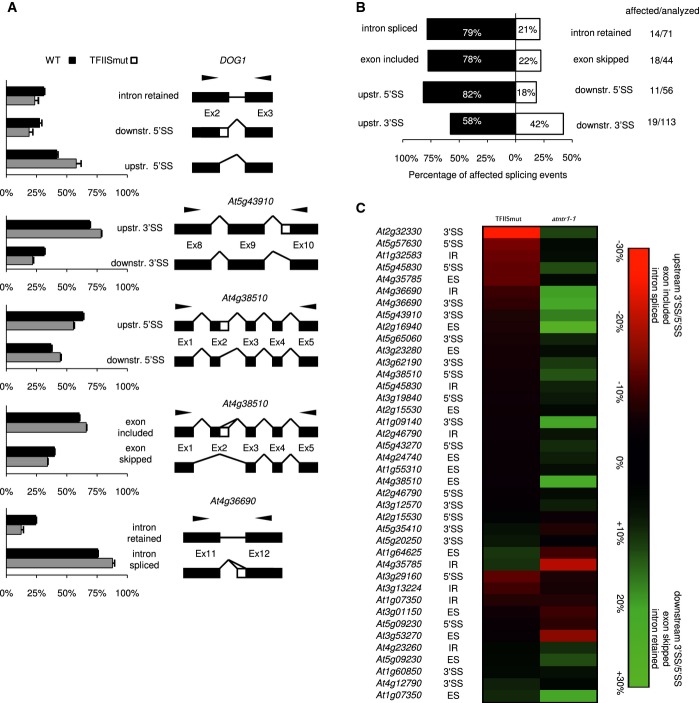
We expected that tfIIs knockout plants would show enhanced pausing of PolII, which in turn would be reflected in alternative splice site selection. Our splicing analysis in tfIIs knockout showed only mild defects in splicing, with 10 of 284 splicing events (including the set analysed for AtNTR1) being affected (Supplementary Fig S7D, Supplementary Table S5). We therefore constructed an enhanced TFIIS mutant in Arabidopsis (TFIISmut). To this end, two key amino acids in the TFIIS trigger loop were mutated, as described previously for yeast (Sigurdsson et al, 2010) (Supplementary Fig S7A). These mutations result in a protein that not only is unable to activate PolII endonucleolytic cleavage but also blocks it, as was shown in vitro (Sigurdsson et al, 2010). We were unable to recover any transformants when TFIISmut construct was expressed the in the tfIIs knockout. This suggests that PolII endonucleolytic cleavage is required for the viability of Arabidopsis, which is in agreement with findings concerning Saccharomyces cerevisiae (Sigurdsson et al, 2010). It was however possible to obtain viable transformants when TFIISmut construct was expressed in the WT plants, as in the case of yeast. These transgenic plants showed a range of developmental defects, including leaf serration and reduced steam elongation (Supplementary Fig S7B). We conclude that, similar to S. cerevisiae, the TFIISmut protein shows a dominant-negative phenotype.
Next, splicing analysis was repeated in TFIISmut using the same set of splicing events that were analysed for the tfIIs knockout. We found that 62 of 284 alternative splicing events were altered (Fig6, Supplementary Fig S7, Supplementary Table S5). For the majority of these cases, including DOG1, preferential selection of the upstream 5′ splice site was observed (nine of 11 cases, Fig6A and B). In addition, a strong tendency towards enhanced intron splicing in the case of intron retention events (11 of 14) and enhanced exon inclusion in the case of exon skipping (14 of 18 events) was observed (Fig6B). This type of bias towards upstream splice sites selection, enhanced intron splicing and exon retention has been postulated to result from the kinetic coupling of splicing and transcriptional elongation (De la Mata et al, 2003, 2011). In summary, of 62 affected alterative splicing events in TFIISmut, 45 changed in the direction predicted by the kinetic model. Our data do not explicitly prove the existence of kinetic coupling of splicing and transcription elongation in plants, but is consistent with this model.
Figure 6.
- TFIISmut shows upstream splice site selection. The splicing was assessed by RT–PCR and capillary electrophoresis in 3-week-old soil grown plants. The chart represents the average relative contribution of the mRNA forms found in the total pool of amplified products. The error bars represent ± SD (n = 3). To the right of the charts, the structures of the examined transcripts are shown. The black boxes, white boxes and black lines represent constitutive exons, alternative regions and introns, respectively. The black arrows show the locations of the primers. Representative splicing assays are shown.
- Directionality of splice site selection in TFIISmut. For each type of alternative splice event, the black and white boxes show the contributions of opposite direction splicing events. The numbers represent the percentage of splice events supporting the direction of the splice site event change (also shown on horizontal axis). The numbers on the right-hand panel represent the number of affected splicing events versus total number of splicing events analysed. The white bars represent downstream 3′ and 5′ splice site selection (3′SS/5′SS), exon skipping (ES) and intron retention (IR), while the black bars represent upstream 3′/5′ splice site selection (3′SS/5′SS), exon inclusion (ES) and intron splicing (IR).
- TFIISmut and the atntr1 mutant have opposite splicing phenotypes. Heat map with scale representing the absolute difference between each respective mutant and Col-0 in alternative splice site usage. The colours represent the direction of the events as described on the axis. Each splicing event is labelled with the gene name and the type of alternative splicing event. The 3′SS/5′SS notation signifies upstream/downstream 5′/3′ splice site selection; ES marks exon skipping or exon inclusion; and IR represents intron retention or intron splicing.
Most importantly, the directionality of the splice site selection bias in the TFIISmut was predominantly opposite to the changes observed in atntr1-1 mutant (compare Fig6B and Fig1E). A total of 72.5% of the splicing events changed in both TFIISmut and atntr1 were affected in opposite directions (Fig6C). These data corroborate our interpretation of directionality in atntr1 alternative splice site selection as a manifestation of PolII elongation defects.
PolII profiling reveals an enhanced PolII pausing at affected introns in TFIISmut
The opposite splice site selection in TFIISmut and atntr1 is consistent with our model of NTR1-dependent transcriptional pausing at the splice sites. It also suggested that TFIIS mutation leads to enhanced PolII pausing at the splice sites. The consequence of this pausing would be manifested by the preference for upstream splice site selection in TFIISmut. To investigate this, a set of six genes was chosen. Those genes are direct targets of AtNTR1 and are oppositely spliced in atntr1-1 and TFIISmut (5 of 6). PolII occupancy in this set of genes was investigated in TFIISmut and atntr1-1 backgrounds. We found that TFIISmut consistently showed higher total PolII occupancy in our assay at the alternatively spliced junction (five of six genes tested) (Supplementary Fig S8). This result showed that the splicing defects observed in TFIISmut are at least correlated with increased pausing of PolII in the context of affected introns.
Previous reports indicated that aberrant splicing leads to the accumulation of PolII at introns. This paused PolII is phosphorylated at Ser5 on the CTD tail by an uncharacterised kinase in yeast (Alexander et al, 2010). Consequently, the phosphorylation status of the PolII CTD tail at Ser5 was analysed in atntr1-1 and TFIISmut plants at our target genes. Indeed, the polymerase showed a predicted change in Ser5 phosphorylation that followed the changes observed in the total PolII level, suggesting that the majority of polymerase paused at those introns was Ser5-phosphorylated (Supplementary Fig S8).
Inhibiting transcription elongation by compromising PolII endonucleolytic cleavage or 6AU treatment partially reverses atntr1 splicing defects
Next, we wanted to substantiate model in which atntr1 splicing changes are a consequence of PolII elongation defects caused by the lack of AtNTR1. We assumed that if this model is correct, then slowing down PolII should reverse some of atntr1 splicing defects. Analysis of splicing in atntr1-1TFIISmut double mutant revealed a clear but only partial reversal of atntr1 splicing for some of the studied genes. Two of five genes studied including DOG1 showed a shift in splice site selection towards the WT in the double mutant (Supplementary Fig S9, Supplementary Table S6). As only partial reversal at two out of five genes tested (or three out of 10 splicing isoforms changed in single mutants with P-value < 0.05 and delta ≥ 5%) could be observed, we extended our set to 45 splicing isoforms (in 14 different genes). Of those 45 analysed, 18 did not show any reversal in the atntr1-1TFIISmut double mutant compared to atntr1-1 and 27 did show partial reversal of splicing (Supplementary Fig S9). Ten of those 27 changed events showed significant change with P-value < 0.05 and delta ≥ 5% when compared directly between the atntr1-1TFIISmut and atntr1-1 single mutant. TFIISmut expression analysis confirmed that the double mutant expressed our transgene at similar level to WT plants (Supplementary Fig S9C).
To further complement those observations, we have used 6AU to challenge transcription elongation in plants and analysed the alternative splicing of a selected set of genes. We have found that 6AU and MPA clearly affect splice site selection in plants. For some of the genes analysed, the pattern is opposite to pattern in atntr1 mutant (three of six) and agree with the prediction of the kinetic coupling model (Supplementary Fig S10). What is more, analysis of atntr1 mutant treated with 6AU showed partial or full reversal of the atntr1 splicing defects, for events where 6AU effect in WT plants is minimal (compare column 1 and 2 in Supplementary Fig S10B). Comparison of atntr1 and atntr1 treated with 6AU leads to identification of 12 significantly changed splicing events. Ten of those events are partially or fully reversed in the atntr1 6AU-treated plants, one event changed in the same direction as in atntr1 compared to WT and 1 event was not changed in atntr1 compared to WT so cannot be classified (Supplementary Fig S10B, third column, events labelled with stars indicate t-test P-value < 0.01).
We therefore concluded that some of the splicing events altered in atntr1 are likely to be a consequence of elongation defects and as such can be reversed by interference with PolII elongation by 6AU or inhibition of PolII endonucleolytic cleavage.
atntr1 shows low sensitivity to 6AU-mediated grow inhibition
Our data provide several lines of evidence for the role of atntr1 in transcription elongation control at splice sites. In yeast, viability test in the presence of 6AU has been extensively used to characterise transcription elongation deficient mutants (Riles et al, 2004). 6AU treatment leads to reduction in in vivo nucleotides levels that causes transcriptional elongation to be more dependent on a fully functional RNA polymerase (Exinger & Lacroute, 1992). WT, tfIIs and atntr1 mutants were subjected to the 6AU treatment. Phenotype analyses showed that tfIIs mutant is indeed highly sensitive to 6AU as expected from numerous reports in yeast. In contrast, atntr1 is strongly resistant to growth inhibition by 6AU (Supplementary Fig S11). The molecular mechanism of 6AU sensitivity in plants has not been studied but the opposite sensitivity of atntr1 and tfIIs mutants to 6AU is reminiscent of opposite directionality in alternative splice site selection and PolII occupancy defects. We therefore interpret the lower sensitivity of atntr1 to 6AU as an indication of AtNTR1 negative role in transcription elongation control in plants.
Discussion
NTR1 was initially characterised as a spliceosomal disassembly factor, and substantial evidence from both humans and yeasts supports its crucial function in this process (Tsai, 2005; Yoshimoto et al, 2014). In Arabidopsis, STILP1 has been described as a NTR1 homologue required for the correct splicing of a range of targets (Jones et al, 2012). Here, we report that AtNTR1 (STILP1) mutants in Arabidopsis, in addition to circadian clock defects, show a pleiotropic phenotype, including weaker seed dormancy and a concomitant reduction in the expression of a key seed dormancy regulator, DOG1.
NTR1 function in splicing
In agreement with the predicted function of NTR1 in splicing, we found that atntr1 mutants show splicing defects in a wide range of genes, including DOG1, where we observe changes in alternative splicing. Although the formal role of NTR1 in spliceosomal disassembly in Arabidopsis thaliana remains unproven, we provide evidence for the interaction of Arabidopsis NTR1 with U6 snRNP, which is consistent with its function in U6 recycling in yeast and humans (Boon et al, 2006; Yoshimoto et al, 2014).
We show evidence for in vivo, direct interaction between AtNTR1 and GCFC domain-containing protein ILP1, in agreement with data for human homologue of NTR1. Interestingly, both Arabidopsis and human NTR1-interacting GCFC proteins are associated directly with gene promoters and regulate gene expression (Yoshizumi et al, 2006; Diao et al, 2012). Therefore, one possibility is that GCFC domain-containing proteins provide target specificity for NTR1 proteins. However, our data point towards strong interdependence of the NTR1 and GCFC proteins in efficient splicing, which is in disagreement with this model and suggests a role of GCFC proteins as spliceosomal factors.
Analysis of NTR1-dependent splice sites revealed that NTR1 is predominantly required for efficient splicing of strong, consensus-like, alternative splice sites. The dependence of strong alternative splice sites on NTR1 may be explained by the U1/U6 exchange defect (Konforti et al, 1993). It is interesting to note that the difference between NTR1-dependent and NTR1-independent splice sites lays in nucleotides forming extended interactions with U1 but not U6. Therefore, the NTR1-dependent splice sites are more efficiently bound by the U1 and may require a relatively high U6 levels or remodelling activity to exchange the U1 for the U6 RNA. Defects in NTR1 in yeast have been shown to lead to reduction in free U6 levels, providing an explanation for the NTR1 specificity we observe (Boon et al, 2006).
NTR1 function in PolII transcription
Beside the involvement of NTR1 in splicing, we provide several lines of evidence for additional transcription-related functions of NTR1.
Careful analysis of atntr1 splicing defects, including splicing defects on DOG1, revealed a bias in alternative splice site selection that is indicative of fast PolII elongation across splice sites. AtNTR1 ChIP data showed that NTR1 is physically located at the target splice sites. Furthermore, we found that its deficiency leads not only to splicing defects but also to locally reduced PolII levels at those splice sites. This has been interpreted by us as faster transcription elongation across those sites. Several lines of evidence corroborate this interpretation. First, the localised PolII occupancy decrease would be difficult to reconcile with a transcriptional change, where PolII levels should be altered globally and not locally. Second, the opposite change in PolII occupancy and directionality of alternative splice site selection between TFIISmut and atntr1 suggests that AtNTR1 has opposite function to TFIISmut in transcription elongation control. Consistent with this interpretation, our transgenic analyses clearly showed AtNTR1-dependent PolII accumulation on strong alternative splice sites. In addition, the reduced sensitivity of atntr1 mutant to 6AU-mediated grow inhibition supports the putative role of NTR1 in transcription elongation control.
The directionality in alternative splice site selection observed in atntr1-, TFIISmut- and 6AU/MPA-treated plants is predominantly consistent with kinetic coupling of transcription and splicing (De la Mata et al, 2003). The kinetic coupling has to our knowledge never been shown to operate in plants. Although our data do not provide a direct confirmation of the kinetic coupling operating in plants, they are consistent with this interpretation.
Splicing analysis in atntr1TFIISmut double mutant showed that some of the atntr1 splicing defects including defects observed at the DOG1 gene can be reversed. Similar reversal of atntr1 splicing defects was observed after atntr1 treatment with 6AU, suggesting that this reversal can be attributed to slow transcription elongation. This can be viewed as a compensation of bona fide spliceosomal defects by the extension of the time window for splicing. The other possibility, that we favour, is that some of the splicing defects in atntr1 are due to faster transcription elongation. Therefore, slowing down of the elongation rate by 6AU or interference with PolII endonucleolytic cleavage (TFIISmut) reverses those splicing defects. Our splicing assay allows for the simultaneous detection of several missplicing events of one gene. Interestingly, in the case of DOG1 and At4g36690, the splice site selection event showed partial reversal in atntr1TFIISmut double mutant background (Supplementary Fig S9A). However, the intron retention event was fully insensitive to TFIISmut in At4g36690 or not significantly changed in DOG1, even though it was altered by TFIISmut in WT backgrounds (Supplementary Fig S9A). The occurrence at the same intron of splicing events that can and cannot be reversed by TFIISmut in the atntr1 background, even though they showed same sensitivity to TFIISmut in the WT background, suggests to us that the reversible splicing events are a consequence of elongation defects in atntr1 rather than the spliceosome dysfunction.
In yeast, introns are sites of transient PolII pausing induced by splicing itself rather than recruitment of the spliceosome (Alexander et al, 2010). This pausing has been proposed to constitute a quality checkpoint for splicing, which provides feedback on transcription (Chathoth et al, 2014). Recently, the role of CUS2 protein as a transcriptional roadblock has been established (Chathoth et al, 2014). We have not addressed the role of CUS2 in transcriptional pausing in Arabidopsis, but our data provide evidence for an analogous role of AtNTR1. The role of NTR1 in transcription elongation control in yeast has also not been addressed. Additionally, yeast NTR1, in contrast to all other higher eukaryotes, lacks several protein domains. One interpretation is that in higher eukaryotes, the increased splicing complexity, including occurrence of alternative splicing, has led to the development of additional splicing-associated transcriptional checkpoints, including the AtNTR1 dependent.
Our chemical and genetic alteration of splicing failed to reproduce the change in PolII levels observed in the case of atntr1 across the splice sites. Data from yeasts and humans show that herboxidiene and both tested splicing mutants work at different stages of splicing than NTR1. This together suggests that the localised reduction in PolII observed in atntr1 is not a consequence of inefficient splicing but rather is a specific consequence of NTR1 deficiency. On the other hand, the minigene analysis showed that the NTR1-dependent PolII pausing requires a strong alternative splice site. This indicates that the strong alternative splice sites are a prerequisite for NTR1 activity both in terms of controlling splicing and in terms of controlling PolII levels at the splice sites. Our data suggest that NTR1 helps to create a pause site for elongating RNA polymerase II at alternative introns to further assist their splicing. This interpretation is consistent with the proposed role of NTR1 in quality proofreading of splicing (Pandit et al, 2006; Koodathingal et al, 2010). Although we acknowledge that a direct assessment of PolII kinetics at the alternatively spliced exons would be required to fully validate our model, we think that a control of transcription elongation at the alternative splice sites by AtNTR1 is the most parsimonious explanation of our data. Given the involvement of H2B ubiquitin transferases homologue in Dog1 regulation (Liu et al, 2007), it would be interesting to see whether it is also involved in the regulation of transcription elongation across alternative splice sites in Arabidopsis.
Materials and Methods
Plant materials, constructs, Arabidopsis transgenic lines and seed dormancy test
Wild-type Col-0 plants, atntr1-1 (SALK_073187), atntr1-2 (GABI_852B07), tfIIs-2 (SALK_027259), ilp1-1 (SALK_030650), ilp1-2 (SALK_135563), dog1-1 (SALK_000867) mutant alleles were used. PCR-based site-directed mutagenesis (Ho et al, 1989) was carried out to generate TFIISmut construct as shown in Supplementary Fig S4, under a 2,390-bp native promoter in pCambia1300. Plants were transformed as described (Logemann et al, 2006). NTR-GFP lines were created by the transformation (Logemann et al, 2006) of atntr1-1 with ATNTR1 genomic fragment including 2,070-bp promoter cloned into pCambia1300, fused in frame with C-terminal GFP-tag. Plants were grown (Sanyo for plates or Conviron walk in chamber for soil) under controlled environmental parameters: 70% humidity, temperature 22°C and 16-h light/8-h dark photoperiod regime at 150–200 mE/m2. For seed dormancy test, freshly harvested seeds were sterilised with 1% NaClO for 10 min and washed with distilled water three times, and seeds were then sown evenly on MS agar plate. Germination rate was scored daily until all genotypes reached 100% germination.
RNA extraction and RT–qPCR
Fourteen-day-old seedlings grown on MS plate and 16-day-old siliques were harvested, frozen in liquid nitrogen immediately and stored at −80°C. RNA was extracted by hot phenol method (Shirzadegan et al, 1991). RevertAid Kit (Thermo Scientific) was used for RT with 5 μg of total RNA, after DNase treatment TURBO DNA-free (Life Technologies), and oligodT primer. One microlitre of RT reaction mix was used for qPCR with SYBR Green I Master (Roche) using LightCycler® 480 Instrument.
Immunoprecipitation and mass spectrometry
AtNTR1-GFP lines were used for immunoprecipitation with GFP-Trap agarose beads (Chromotek), following user manual. Eluted proteins were precipitated by acetone and digested by trypsin (Thermo Scientific). Mass spectrometry was performed in Institute of Biochemistry and Biophysics proteomics facility. Peptide mixtures were applied to RP-18 pre-column on UPLC system (NanoAcquity; Waters) using water containing 0.1% FA as a mobile phase followed by a nano-HPLC RP-18 column (75 μM; Waters) using ACN gradient (0–35% ACN in 160 min) in the presence of 0.1% FA at a flow rate of 250 nl/min. The column outlet was coupled directly to the ion source of Orbitrap Velos mass spectrometer (Thermo). The acquired MS/MS data were pre-processed with Mascot Distiller software (v. 2.4.3; MatrixScience) and a search was performed with the Mascot Search Engine (MatrixScience, Mascot Server 2.4) against the TAIR10 database (35,386 sequences; 14,482,855 residues). Mascot search settings were as follows: enzyme: trypsin, missed cleaveages: 0, fixed modifications: carbamidomethyl (C), variable modifications: oxidation (M).
Immuno-co-localisation
DNDYEGGRWEGDEFVYC and DMIDEDVEVRGGLGIGC peptides were synthesised to generate rabbit anti-AtNTR1 polyclonal antibody (Eurogentec). Immuno-co-localisation was performed as described (Zhang et al, 2013). Nuclei were fixed with 4% paraformaldehyde and blocked 15 min with 0.05% acBSA (Aurion, the Netherlands) in PBS buffer. Then, nuclei were incubated in double-labelling reactions with primary antibodies: anti-AtNTR1 diluted 1:200 and mouse IgG (4H8) antibody (Covance) diluted 1:100 or mouse IgG anti-SC35 (Sigma) diluted 1:100, or mouse IgM anti-RNA polymerase II H14 (recognises the phosphoserine 5 version of RNA PolII, PolIIA) or mouse IgM anti-RNA polymerase II H5 (recognises the phosphoserine 2 version of RNA PolII, PolIIO) (Covance) diluted 1:100 in PBS buffer with 0.05% ac BSA overnight at 8°C. Nuclei were then washed in PBS and incubated in secondary antibodies: Alexa Fluor 594 goat anti-rabbit (1:500, Invitrogen) and Alexa Fluor 488 goat anti-mouse IgG (1:1,000, Invitrogen) or Alexa Fluor 488 goat anti-mouse IgM (1:500; Invitrogen), at 37°C for 1 h. DNA was counterstained with 4,6-diamidino-2-phenylindole (DAPI; Fluka). The results were registered with Leica SP8 confocal and processed by Adobe PhotoShop.
BiFC assay and yeast two-hybrid assay
ORF of AtNTR1 and ILP1 were cloned to pSPYNE173 and pSPYCE(M) (Waadt et al, 2008), and protoplast isolation and transformation were performed as described (Wu et al, 2009). Fluorescence images were acquired by Nikon Eclipse TE2000-E inverted microscope and processed by Nikon EZ-C1 software. For yeast two-hybrid assay, The AtNTR1 and ILP1 ORF were amplified from cDNA and fused in frame to pGBT9 Gal4 binding domain (BD) and pGAD424 Gal4 activating domain (AD) (Clontech). The yeast strain AH109 was co-transformed with corresponding vector and grown on dropout medium –LT (leucin and trypsin deficient) for selection at 28°C. Serial decimal dilutions were used for low stringency selection on –LHT (leucin, trypsin and histidine deficient) plates and high stringency selection on –LHTA (leucin, trypsin, histidine and adenine deficient) plates.
Splicing analysis
Splicing analysis was performed using 6-FAM (Sigma-Aldrich) labelled forward primer and capillary electrophoresis on ABI3730 DNA Analyzer (Life Technologies) as described (Simpson et al, 2007; Raczynska et al, 2014). Primer sequences for alternative spliced mRNA are given in Supplementary Table S7. Peak areas for each alternative variant were analysed using PeakScanner (Life Technologies), and contribution of each alternative isoforms was calculated as percentage of total isoforms detected. Means and standard deviations were calculated for three separate biological repetitions. Changes in AS events were considered significant if P-value < 0.05 (t-test) and change ≥ 5%. AtNTR1 splicing has been analysed using alternative splicing panel (Ji et al, 2007). We therefore reanalysed a selected set of 144 alternative splicing events chosen to be robust in both 2- and 3-week-old seedlings as described in figure legends. DOG1 alternative splicing analysis shown in Supplementary Fig S1D was performed as described in Schwab (2008).
Consensus analysis
Splicing sites of affected and unaffected introns from our data set have been aligned, and Web logo was created using Weblogo3 generator (Schneider and Stephens 1990; Crooks, 2004). Arabidopsis consensus was created using introns from AATDB version 3-5. For AtNTR1-dependent and AtNTR1-independent intron analyses, only introns with alternative 5′ and 3′ site selection were used. Statistical significance was calculated using Fisher's two-tailed exact test based on frequencies of A/G A dinucleotides at positions +3/+4 in changed and unchanged introns with alternative splice sites (Fisher 1922).
Chemical modulation of splicing and elongation
Plant were grown in liquid culture in 1/2MS with sucrose 15 g/l, and 2-week-old plants were incubated for 3 h before harvest. 6AU (10 mg/l), MPA (5 mg/l) or herboxidiene (1.5 mg/l) were used at final concentrations shown. For 6AU viability assay, plants were grown on MS plates with 6AU (0.5 mg/l).
Chromatin immunoprecipitation (ChIP)
Chromatin immunoprecipitation was performed as described (Bowler et al, 2004) with IP buffer prepared as described (Kaufmann et al, 2010). Chromatin was sonicated at 4°C with a Diagenode Bioruptor at high intensity for 10 min (30 s on/30 s off). Antibodies: total PolII (Agrisera AS11 1804), P-Ser5 (Santa Cruz sc-47701) or peptide purified AtNTR1 antibodies described above were used with Dynabeads Protein G (Life Technologies). Chelex (Biorad) was used for de-crosslinking as described (Nelson et al, 2006). No antibody control was used to determine nonspecific background. Percentage of input was calculated for each sample using quantitative PCR. Primer sequences for qPCR are given in Supplementary Table S8. No antibody control showed signal an order of magnitude lower than the performed side-by-side PolII ChIP experiment (Supplementary Fig S8 bottom panel).
RNA immunoprecipitation (RIP)
Nuclear fraction was purified as for ChIP. RNA-specific steps were performed as described (Rowley et al, 2013). NTR1 complexes were immunoprecipitated using anti-GFP antibody (Chromotek gt-250). RNA was treated with DNase and reverse-transcribed using random primers (Thermo Scientific) and SuperScriptIII RT (Life Technologies).
Acknowledgments
We thank Professor John Brown and Dr Craig Simpson for help with alternative splicing panel, Thomas R. Webb for his excellent advice with selecting splicing modulator and Zbigniew Pietras for critical reading of the manuscript. This project was supported by National Science Centre (N N301 388239 and N N301 269237 to SS, UMO-2011/01/M/NZ2/01435 to AJ, UMO-2011/03/N/NZ2/03070 and UMO-2014/12/T/NZ2/00246 to JD), National Centre for Research and Development (LIDER/22/139/L-1/09/NCBiR/2010 to SS and YG) and Foundation for Polish Science (TEAM2010-5/9 to SS and GB and MPD/2010/7 for JD).
Author contributions
JD performed splicing platform analysis, PolII ChIPs, RIP; AK, DS and YG performed the immunolocalisation; GB performed Y2H; YG obtained the TFIISmut plants and performed all other experiments; YG, JD, AJ and SŚ designed the experiments; AJ and SŚ analysed the data; and SŚ wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Figure S9
Supplementary Figure S10
Supplementary Figure S11
Supplementary Tables S1–S8
Supplementary Figure Legends
Source Data for Supplementary Figure S5
Source Data for Supplementary Figure S6
Source Data for Supplementary Figure S8
Review Process File
Source Data for Figure 4
Source Data for Figure 5
References
- Agafonov DE, Deckert J, Wolf E, Odenwälder P, Bessonov S, Will CL, Urlaub H, Lührmann R. Semiquantitative proteomic analysis of the human spliceosome via a novel two-dimensional gel electrophoresis method. Mol Cell Biol. 2011;31:2667–2682. doi: 10.1128/MCB.05266-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RD, Innocente SA, Barrass JD, Beggs JD. Splicing-dependent RNA polymerase pausing in yeast. Mol Cell. 2010;40:582–593. doi: 10.1016/j.molcel.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsché E, Yaniv M, Muchardt C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat Struct Mol Biol. 2005;13:22–29. doi: 10.1038/nsmb1030. [DOI] [PubMed] [Google Scholar]
- Bentsink L, Jowett J, Hanhart CJ, Koornneef M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:17042–17047. doi: 10.1073/pnas.0607877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon K-L, Auchynnikava T, Edwalds-Gilbert G, Barrass JD, Droop AP, Dez C, Beggs JD. Yeast Ntr1/Spp382 mediates Prp43 function in postspliceosomes. Mol Cell Biol. 2006;26:6016–6023. doi: 10.1128/MCB.02347-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Benvenuto G, Laflamme P, Molino D, Probst AV, Tariq M, Paszkowski J. Chromatin techniques for plant cells. Plant J. 2004;39:776–789. doi: 10.1111/j.1365-313X.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- Braberg H, Jin H, Moehle EA, Chan YA, Wang S, Shales M, Benschop JJ, Morris JH, Qiu C, Hu F, Tang LK, Fraser JS, Holstege FCP, Hieter P, Guthrie C, Kaplan CD, Krogan NJ. From structure to systems: high-resolution, quantitative genetic analysis of RNA polymerase II. Cell. 2013;154:775–788. doi: 10.1016/j.cell.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody Y, Neufeld N, Bieberstein N, Causse SZ, Böhnlein E-M, Neugebauer KM, Darzacq X, Shav-Tal Y. The in vivo kinetics of RNA polymerase II elongation during co-transcriptional splicing. PLoS Biol. 2011;9:e1000573. doi: 10.1371/journal.pbio.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S. Progression through the RNA Polymerase II CTD Cycle. Mol Cell. 2009;36:541–546. doi: 10.1016/j.molcel.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanarat S, Seizl M, Strässer K. The Prp19 complex is a novel transcription elongation factor required for TREX occupancy at transcribed genes. Genes Dev. 2011;25:1147–1158. doi: 10.1101/gad.623411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chathoth KT, Barrass JD, Webb S, Beggs JD. A splicing-dependent transcriptional checkpoint associated with prespliceosome formation. Mol Cell. 2014;53:779–790. doi: 10.1016/j.molcel.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ACM, Cramer P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature. 2011;471:249–253. doi: 10.1038/nature09785. [DOI] [PubMed] [Google Scholar]
- Close P, East P, Dirac-Svejstrup AB, Hartmann H, Heron M, Maslen S, Chariot A, Söding J, Skehel M, Svejstrup JQ. DBIRD complex integrates alternative mRNA splicing with RNA polymerase II transcript elongation. Nature. 2012;484:386–389. doi: 10.1038/nature10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvitkovic I, Jurica MS. Spliceosome database: a tool for tracking components of the spliceosome. Nucleic Acids Res. 2013;41:D132–D141. doi: 10.1093/nar/gks999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Mata M, Alonso CR, Kadener S, Fededa JP, Blaustein M, Pelisch F, Cramer P, Bentley D, Kornblihtt AR. A slow RNA polymerase II affects alternative splicing in vivo. Mol Cell. 2003;12:525–532. doi: 10.1016/j.molcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- De la Mata M, Muñoz MJ, Alló M, Fededa JP, Schor IE, Kornblihtt AR. RNA polymerase II elongation at the crossroads of transcription and alternative splicing. Genet Res Int. 2011;2011:1–9. doi: 10.4061/2011/309865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y, Guo X, Li Y, Sun K, Lu L, Jiang L, Fu X, Zhu H, Sun H, Wang H, Wu Z. Pax3/7BP Is a Pax7- and Pax3-binding protein that regulates the proliferation of muscle precursor cells by an epigenetic mechanism. Cell Stem Cell. 2012;11:231–241. doi: 10.1016/j.stem.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Dujardin G, Lafaille C, de la Mata M, Marasco LE, Muñoz MJ, Le Jossic-Corcos C, Corcos L, Kornblihtt AR. How slow RNA polymerase II elongation favors alternative exon skipping. Mol Cell. 2014;54:683–690. doi: 10.1016/j.molcel.2014.03.044. [DOI] [PubMed] [Google Scholar]
- Dutertre M, Sanchez G, De Cian M-C, Barbier J, Dardenne E, Gratadou L, Dujardin G, Le Jossic-Corcos C, Corcos L, Auboeuf D. Cotranscriptional exon skipping in the genotoxic stress response. Nat Struct Mol Biol. 2010;17:1358–1366. doi: 10.1038/nsmb.1912. [DOI] [PubMed] [Google Scholar]
- Exinger F, Lacroute F. 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr Genet. 1992;22:9–11. doi: 10.1007/BF00351735. [DOI] [PubMed] [Google Scholar]
- Filichkin SA, Priest HD, Givan SA, Shen R, Bryant DW, Fox SE, Wong W-K, Mockler TC. Genome-wide mapping of alternative splicing in Arabidopsis thaliana. Genome Res. 2009;20:45–58. doi: 10.1101/gr.093302.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85:87–94. [Google Scholar]
- Grasser M, Kane CM, Merkle T, Melzer M, Emmersen J, Grasser KD. Transcript elongation factor TFIIS is involved in Arabidopsis seed dormancy. J Mol Biol. 2009;386:598–611. doi: 10.1016/j.jmb.2008.12.066. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Miura T, Kuzuya K, Inoue A, Won Ki S, Horinouchi S, Yoshida T, Kunoh T, Koseki K, Mino K, Sasaki R, Yoshida M, Mizukami T. Identification of SAP155 as the Target of GEX1A (Herboxidiene), an Antitumor Natural Product. ACS Chem Biol. 2011;6:229–233. doi: 10.1021/cb100248e. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Howe KJ, Kane CM, Ares M., Jr Perturbation of transcription elongation influences the fidelity of internal exon inclusion in Saccharomyces cerevisiae. RNA. 2003;9:993–1006. doi: 10.1261/rna.5390803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip JY, Schmidt D, Pan Q, Ramani AK, Fraser AG, Odom DT, Blencowe BJ. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res. 2011;21:390–401. doi: 10.1101/gr.111070.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izban MG, Luse DS. The RNA polymerase II ternary complex cleaves the nascent transcript in a 3′–5' direction in the presence of elongation factor SII. Genes Dev. 1992;6:1342–1356. doi: 10.1101/gad.6.7.1342. [DOI] [PubMed] [Google Scholar]
- Ji Q, Huang C-H, Peng J, Hashmi S, Ye T, Chen Y. Characterization of STIP, a multi-domain nuclear protein, highly conserved in metazoans, and essential for embryogenesis in Caenorhabditis elegans. Exp Cell Res. 2007;313:1460–1472. doi: 10.1016/j.yexcr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Jones MA, Williams BA, McNicol J, Simpson CG, Brown JWS, Harmer SL. Mutation of Arabidopsis SPLICEOSOMAL TIMEKEEPER LOCUS1 causes circadian clock defects. Plant Cell. 2012;24:4066–4082. doi: 10.1105/tpc.112.104828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida D, Motoyoshi H, Tashiro E, Nojima T, Hagiwara M, Ishigami K, Watanabe H, Kitahara T, Yoshida T, Nakajima H, Tani T, Horinouchi S, Yoshida M. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat Chem Biol. 2007;3:576–583. doi: 10.1038/nchembio.2007.18. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Muiño JM, Østerås M, Farinelli L, Krajewski P, Angenent GC. Chromatin immunoprecipitation (ChIP) of plant transcription factors followed by sequencing (ChIP-SEQ) or hybridization to whole genome arrays (ChIP-CHIP) Nat Protoc. 2010;5:457–472. doi: 10.1038/nprot.2009.244. [DOI] [PubMed] [Google Scholar]
- Komarnitsky P. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 2000;14:2452–2460. doi: 10.1101/gad.824700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konforti BB, Koziolkiewicz MJ, Konarska MM. Disruption of base pairing between the 5′ splice site and the 5′ end of U1 snRNA is required for spliceosome assembly. Cell. 1993;75:863–873. doi: 10.1016/0092-8674(93)90531-t. [DOI] [PubMed] [Google Scholar]
- Koodathingal P, Novak T, Piccirilli JA, Staley JP. The DEAH box ATPases Prp16 and Prp43 cooperate to proofread 5′ splice site cleavage during pre-mRNA splicing. Mol Cell. 2010;39:385–395. doi: 10.1016/j.molcel.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetti C, Yermolina MV, Sharma LK, Palacios G, Prigaro BJ, Webb TR. Pre-mRNA splicing-modulatory pharmacophores: the total synthesis of herboxidiene, a pladienolide-herboxidiene hybrid analog and related derivatives. ACS Chem Biol. 2014;9:643–648. doi: 10.1021/cb400695j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Koornneef M, Soppe WJJ. The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell. 2007;19:433–444. doi: 10.1105/tpc.106.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann E, Birkenbihl RP, Ulker B, Somssich IE. An improved method for preparing Agrobacterium cells that simplifies the Arabidopsis transformation protocol. Plant Methods. 2006;2:16. doi: 10.1186/1746-4811-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez Y, Brown JWS, Simpson C, Barta A, Kalyna M. Transcriptome survey reveals increased complexity of the alternative splicing landscape in Arabidopsis. Genome Res. 2012;22:1184–1195. doi: 10.1101/gr.134106.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayas RM, Maita H, Semlow DR, Staley JP. Spliceosome discards intermediates via the DEAH box ATPase Prp43p. Proc Natl Acad Sci. 2010;107:10020. doi: 10.1073/pnas.0906022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Wideman M, Makkar N, Tran M, Isaac B, Biest N, Stonard R. Herboxidiene, a new herbicidal substance from Streptomyces chromofuscus A7847. Taxonomy, fermentation, isolation, physico-chemical and biological properties. J Antibiot (Tokyo) 1992;45:914–921. doi: 10.7164/antibiotics.45.914. [DOI] [PubMed] [Google Scholar]
- Mortensen SA, Grasser KD. The seed dormancy defect of Arabidopsis mutants lacking the transcript elongation factor TFIIS is caused by reduced expression of the DOG1 gene. FEBS Lett. 2014;588:47–51. doi: 10.1016/j.febslet.2013.10.047. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Bomsztyk K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc. 2006;1:179–185. doi: 10.1038/nprot.2006.27. [DOI] [PubMed] [Google Scholar]
- Pagani F, Stuani C, Zuccato E, Kornblihtt AR, Baralle FE. Promoter architecture modulates CFTR exon 9 skipping. J Biol Chem. 2003;278:1511–1517. doi: 10.1074/jbc.M209676200. [DOI] [PubMed] [Google Scholar]
- Palusa SG, Ali GS, Reddy ASN. Alternative splicing of pre-mRNAs of Arabidopsis serine/arginine-rich proteins: regulation by hormones and stresses: stress regulation of alternative splicing of SR genes. Plant J. 2007;49:1091–1107. doi: 10.1111/j.1365-313X.2006.03020.x. [DOI] [PubMed] [Google Scholar]
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- Pandit S, Lynn B, Rymond BC. Inhibition of a spliceosome turnover pathway suppresses splicing defects. Proc Natl Acad Sci. 2006;103:13700–13705. doi: 10.1073/pnas.0603188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczynska KD, Stepien A, Kierzkowski D, Kalak M, Bajczyk M, McNicol J, Simpson CG, Szweykowska-Kulinska Z, Brown JWS, Jarmolowski A. The SERRATE protein is involved in alternative splicing in Arabidopsis thaliana. Nucleic Acids Res. 2014;42:1224–1244. doi: 10.1093/nar/gkt894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines D. Elongation factor-dependent transcript shortening by template-engaged RNA polymerase II. J Biol Chem. 1992;267:3795–3800. [PMC free article] [PubMed] [Google Scholar]
- Riles L, Shaw RJ, Johnston M, Reines D. Large-scale screening of yeast mutants for sensitivity to the IMP dehydrogenase inhibitor 6-azauracil. Yeast. 2004;21:241–248. doi: 10.1002/yea.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley MJ, Böhmdorfer G, Wierzbicki AT. Analysis of long non-coding RNAs produced by a specialized RNA polymerase in Arabidopsis thaliana. Methods (San Diego Calif) 2013;63:160–169. doi: 10.1016/j.ymeth.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-André V, Batsché E, Rachez C, Muchardt C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat Struct Mol Biol. 2011;18:337–344. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- Schwab M. Identification of novel seed dormancy mutants in Arabidopsis thaliana and molecular and biochemical characterization of the seed dormancy gene DOG1. 2008. PhD Thesis, Faculty of Mathematics and Natural Sciences, University of Cologne, Cologne.
- Schneider TD, Stephens RM. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimizu K, Kobayashi N, Mizuno D, Natori S. Purification of a factor from Ehrlich ascites tumor cells specifically stimulating RNA polymerase II. Biochemistry (Mosc) 1976;15:5064–5070. doi: 10.1021/bi00668a018. [DOI] [PubMed] [Google Scholar]
- Shirzadegan M, Christie P, Seemann JR. An efficient method for isolation of RNA from tissue cultured plant cells. Nucleic Acids Res. 1991;19:6055. doi: 10.1093/nar/19.21.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature. 2011;479:74–79. doi: 10.1038/nature10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson S, Dirac-Svejstrup AB, Svejstrup JQ. Evidence that transcript cleavage is essential for RNA polymerase II transcription and cell viability. Mol Cell. 2010;38:202–210. doi: 10.1016/j.molcel.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CG, Fuller J, Maronova M, Kalyna M, Davidson D, McNicol J, Barta A, Brown JWS. Monitoring changes in alternative precursor messenger RNA splicing in multiple gene transcripts. Plant J. 2007;53:1035–1048. doi: 10.1111/j.1365-313X.2007.03392.x. [DOI] [PubMed] [Google Scholar]
- Swaraz AM, Park Y-D, Hur Y. Knock-out mutations of Arabidopsis SmD3-b induce pleotropic phenotypes through altered transcript splicing. Plant Sci. Int J Exp Plant Biol. 2011;180:661–671. doi: 10.1016/j.plantsci.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Tannukit S, Wen X, Wang H, Paine ML. TFIP11, CCNL1 and EWSR1 protein-protein interactions, and their nuclear localization. Int J Mol Sci. 2008;9:1504–1514. doi: 10.3390/ijms9081504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai R-T. Spliceosome disassembly catalyzed by Prp43 and its associated components Ntr1 and Ntr2. Genes Dev. 2005;19:2991–3003. doi: 10.1101/gad.1377405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J Cell Mol Biol. 2008;56:505–516. doi: 10.1111/j.1365-313X.2008.03612.x. [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Wen X, Lei Y-P, Zhou YL, Okamoto CT, Snead ML, Paine ML. Structural organization and cellular localization of tuftelin-interacting protein 11 (TFIP11) Cell Mol Life Sci. 2005;62:1038–1046. doi: 10.1007/s00018-005-4547-z. [DOI] [PubMed] [Google Scholar]
- Wu F-H, Shen S-C, Lee L-Y, Lee S-H, Chan M-T, Lin C-S. Tape-Arabidopsis sandwich—a simpler Arabidopsis protoplast isolation method. Plant Methods. 2009;5:16. doi: 10.1186/1746-4811-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto R, Okawa K, Yoshida M, Ohno M, Kataoka N. Identification of a novel component C2ORF3 in the lariat-intron complex: lack of C2ORF3 interferes with pre-mRNA splicing via intron turnover pathway. Genes Cells. 2014;19:78–87. doi: 10.1111/gtc.12114. [DOI] [PubMed] [Google Scholar]
- Yoshizumi T, Tsumoto Y, Takiguchi T, Nagata N, Yamamoto YY, Kawashima M, Ichikawa T, Nakazawa M, Yamamoto N, Matsui M. Increased level of polyploidy 1, a conserved repressor of CYCLINA2 transcription, controls endoreduplication in Arabidopsis. Plant Cell. 2006;18:2452–2468. doi: 10.1105/tpc.106.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C-J, Zhou J-X, Liu J, Ma Z-Y, Zhang S-W, Dou K, Huang H-W, Cai T, Liu R, Zhu J-K, He X-J. The splicing machinery promotes RNA-directed DNA methylation and transcriptional silencing in Arabidopsis. EMBO J. 2013;32:1128–1140. doi: 10.1038/emboj.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Figure S9
Supplementary Figure S10
Supplementary Figure S11
Supplementary Tables S1–S8
Supplementary Figure Legends
Source Data for Supplementary Figure S5
Source Data for Supplementary Figure S6
Source Data for Supplementary Figure S8
Review Process File
Source Data for Figure 4
Source Data for Figure 5