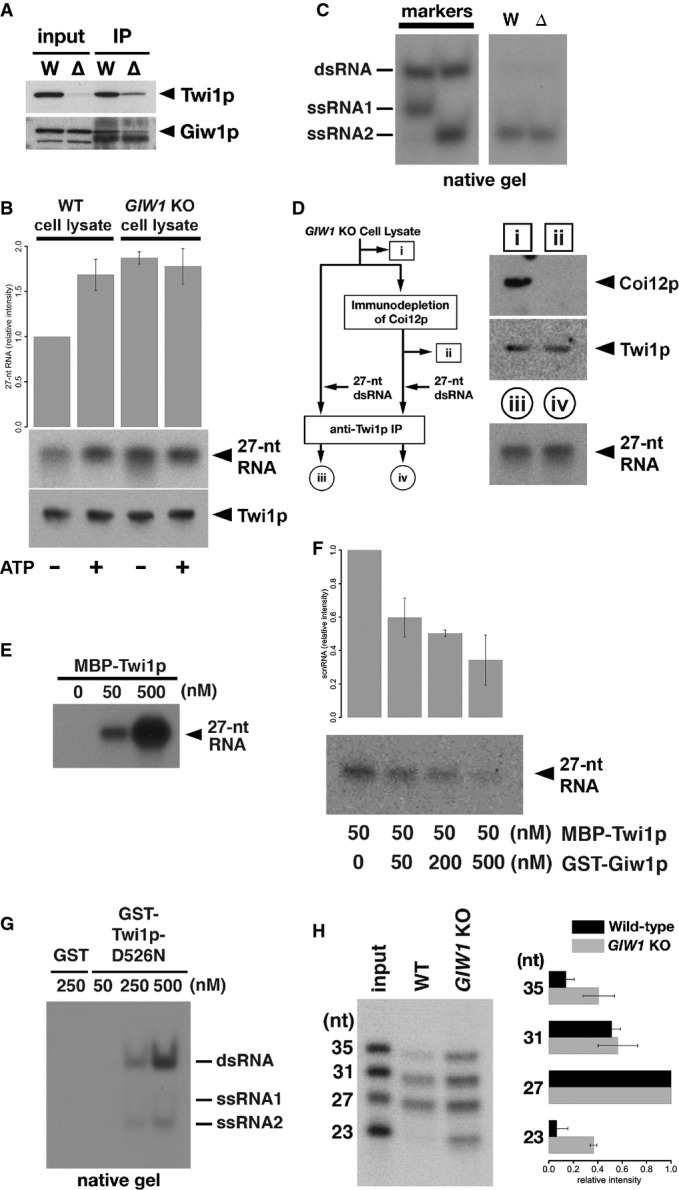
The Twi1p-containing complex was immunoprecipitated using an anti-Twi1p antibody from wild-type (W) and DCL1KO (Δ) cells, and input (left) and precipitated (right) proteins were analyzed by Western blot using an anti-Twi1p antibody (top) and an anti-Giw1p antibody (bottom).
Cell lysates from wild-type (WT) or GIW1KO cells at 3 hpm were incubated with radiolabeled double-stranded 27-nt RNAs and with (+) or without (−) ATP and the ATP regeneration system. The Twi1p-containing complex was immunoprecipitated with an anti-Twi1p antibody, co-precipitated RNAs were separated on a denaturing gel and detected by autoradiography. The intensities of the bands in the different conditions relative to the sample with wild-type cell lysate and without ATP (1st column) are shown on the top. The standard deviation (SD) between technical replicates is indicated. A result of a representative experiment is shown on the bottom.
RNAs were prepared from cell lysates from wild-type (W) or GIW1KO (Δ) cells with ATP and the ATP regeneration system as in (A) but were separated in a native gel. The positions of double-stranded 27-nt RNAs (dsRNA) and each strand of RNA used to form the 27-nt RNA duplex (ssRNA1 and ssRNA2) are indicated at the left.
Coi12p was immunodepleted from GIW1KO cell lysate at 3 hpm, and the lysate was incubated with radiolabeled double-stranded 27-nt RNAs. As a control, GIW1KO cell lysate without Coi12p depletion was used for the loading assay. The protein samples (i, ii) correspond to the lanes shown in the top right panel. Coi12p and Twi1p in the lysates were detected by Western blot. The Twi1p-containing complex was immunoprecipitated with an anti-Twi1p antibody, and co-precipitated 27-nt RNAs were detected by autoradiography. The RNA samples (iii, iv) correspond to the lanes shown in the bottom right panel.
The indicated concentration of MBP-tagged Twi1p (MBP-Twi1p), which was recombinantly expressed in E. coli, was incubated with the radiolabeled double-stranded 27-nt RNAs. Loaded RNA was analyzed by immunoprecipitating MBP-Twi1p with an anti-Twi1p antibody and separated on a denaturing gel, followed by autoradiographic detection.
MBP-Twi1p (50 nM) was incubated with the indicated concentrations of the recombinantly expressed GST-Giw1p and with radiolabeled 27-nt RNA duplexes. Loaded RNA was analyzed as in (C). The intensities of the bands in the different conditions relative to the sample without GST-Giw1p (1st column) are shown on the top. The standard deviation (SD) between technical replicates is indicated. A result of a representative experiment is shown on the bottom.
The indicated concentration of recombinantly expressed GST-tagged Twi1p in which aspartic acid 526 was replaced by asparagine (GST-Twi1p-D526N) or GST alone was incubated with radiolabeled double-stranded 27-nt RNAs. Loaded RNA was analyzed by immunoprecipitating GST-Twi1p-D526N with an anti-Twi1p antibody and separating on a native gel, followed by autoradiographic detection. The positions of double-stranded 27-nt RNAs (dsRNA) and each strand of RNA used to form the 27-nt RNA duplex (ssRNA1 and ssRNA2) are indicated at the right.
Cell lysates from wild-type (WT) or GIW1KO cells at 3 hpm were incubated with a mixture of radiolabeled double-stranded 23-, 27-, 31-, and 35-nt RNAs, ATP, and the ATP regeneration system. The Twi1p-containing complex was immunoprecipitated with an anti-Twi1p antibody, and co-precipitated RNAs were detected by autoradiography. The intensities of each RNA band relative to the 27-nt RNA were normalized to the input sample and are shown on the right. The standard deviation (SD) between technical replicates is indicated. A representative experiment is shown on the left.