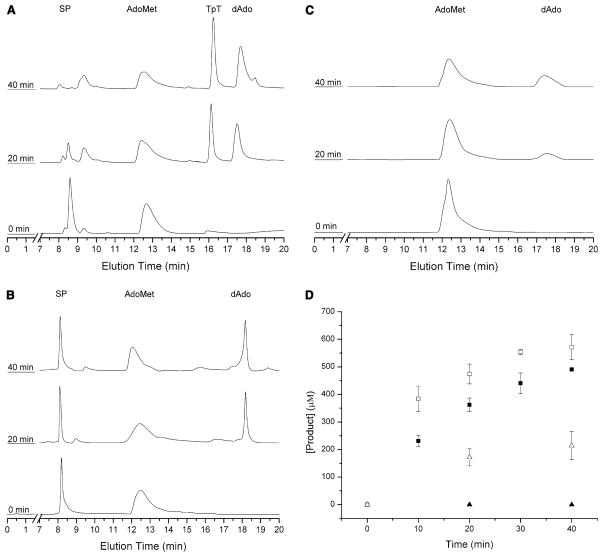
Fig. 8.
High performance liquid chromatography (HPLC) chromatograms demonstrating the time-dependent formation of thymidylyl-(3′–5′)-thymidine (TpT) due to repair of (5R)-SPTpT (a) but not (5S)-SPTpT (b), upon incubation of 500 μM SP with SP lyase (50 μM), AdoMet (1 mM), DTT (5 mM) and dithionite (1 mM) in 17 mM sodium phosphate, 100 mM NaCl, 6 mM KCl, pH 7.5 at 303 K. SPTpT is eluted at 8.5 min (5R) or 8.1 min (5S) and TpT is eluted at 16.2 min under these conditions. HPLC chromatograms of the negative control (lacking substrate) (c) demonstrating the time-dependent cleavage of AdoMet by SP lyase (50 μM) incubated with AdoMet (1 mM), DTT (5 mM) and dithionite (1 mM) in 17 mM sodium phosphate, 100 mM NaCl, 6 mM KCl, pH 7.5 at 303 K. Integration of the TpT, SPTpT, AdoMet, and dAdo peaks allowed for the quantification of each isomer of SP and AdoMet cleavage (d). The repair of (5R)-SPTpT (solid squares) demonstrates the repair over the course of 40 min, whereas assays of (5S)-SPTpT (solid triangles) resulted in no detectable formation of TpT. Formation of dAdo is shown for (5R)-SPTpT (open squares) as well as for (5S)-SPTpT (open triangles)