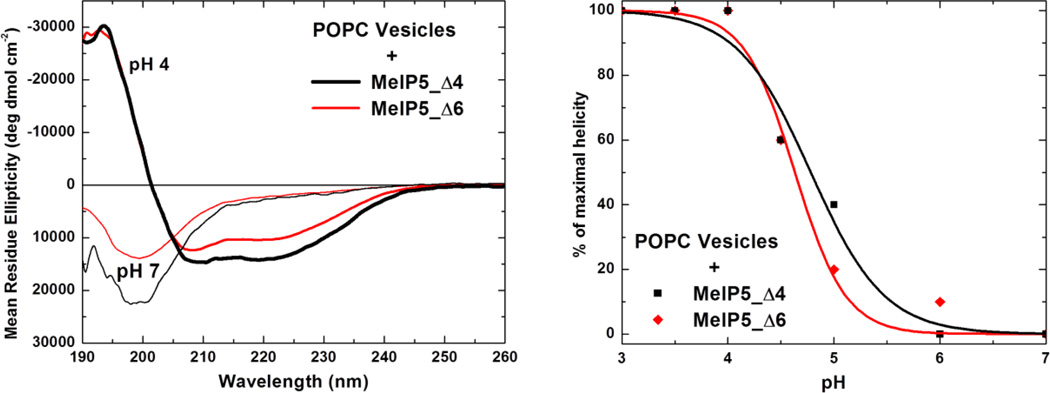
Figure 3.
Secondary structure of MelP5_Δ4 and MelP5_Δ6. Left: The spectra of 100 µM MelP5_Δ4 and MelP5_Δ6 in the presence of 1 mM POPC lipid vesicles, at neutral pH and at acidic pH. At pH 4, the spectra indicate α-helical structure, whereas at pH 7 the spectra are characteristic of random coil structure. Such measurements were carried out as a function of pH and the results are plotted in the right panel. The fraction helix is calculated from the ellipticity at 222 nm and is normalized to the maximal value, which is obtained at pH 4.