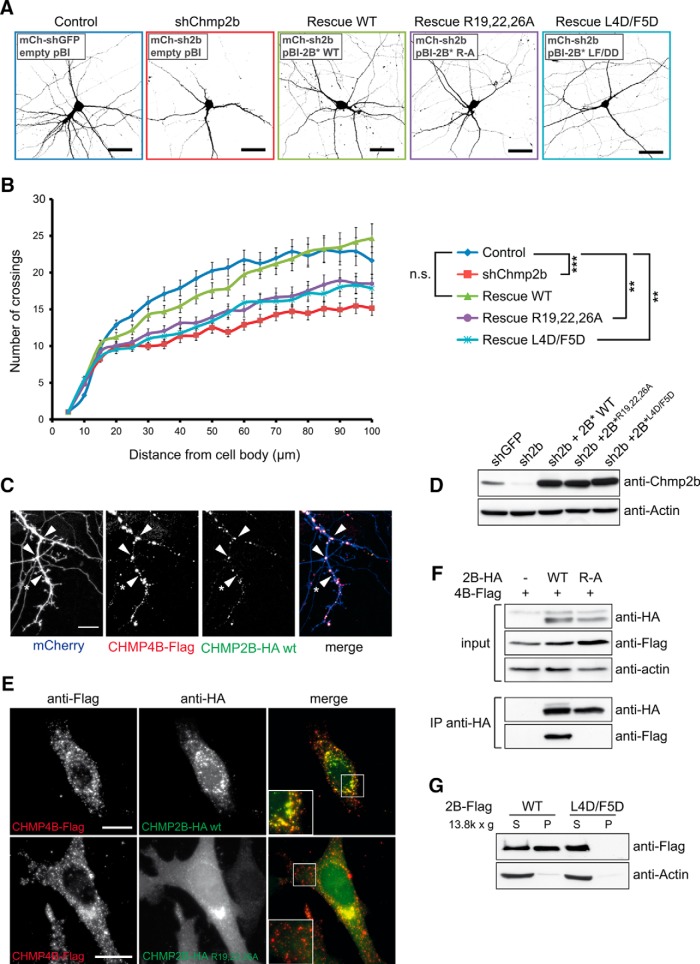
Figure 3.
Chmp2b regulates dendritic morphology in an ESCRT-III-dependent manner. A, Hippocampal neurons were cotransfected at 10 DIV with the indicated mixtures of pSuper-based and rescue (pBI-based) plasmids. pSuper vectors expressed mCherry together with the indicated shRNA [GFP shRNA (shGFP); Chmp2b shRNA (shChmp2b or sh2b)]. Rescue plasmids expressed ZsGreen, alone or together with the indicated version of RNAi-resistant Chmp2b (2B*). Images of doubly transfected neurons at 14 DIV were acquired by confocal microscopy. Depicted is the morphology of representative neurons in each condition, as visualized by mCherry fluorescence (negative contrast, maximum intensity projections). B, Sholl analysis of dendritic arborization in neurons transfected as in A. For each plasmid mix, the graph shows mean numbers of dendritic branches as a function of radial distance (n = 40 neurons per condition). Chmp2b knockdown strongly reduced the distance-dependent increase in branch number (***p = 0.00014 compared with control curve, Kolmogorov–Smirnov test with Bonferroni's correction). Expression of wild-type Chmp2b* restored the control phenotype (p = 1). Chmp4b-binding-defective (Chmp2b*R19/22/26A) and membrane-binding-defective (Chmp2b* L4D/F5D) mutants of Chmp2b were unable to fully restore the control phenotype (**p = 0.004 and p = 0.006, respectively). C, Hippocampal neurons were triply cotransfected at 10 DIV with HA-tagged Chmp2bWT, Flag-tagged Chmp4b, and mCherry plasmids (1 μg of each plasmid). The cells were fixed 24 h later and stained by dual immunofluorescence with anti-HA and anti-Flag antibodies. Confocal microscopy images (z-projections) of dendritic fluorescence are shown for a representative neuron. Chmp2b was recruited to Chmp4b puncta, some of which were found at dendritic branch points (arrowheads) or at the base of spine-looking protrusions (asterisk). Scale bar, 10 μm. D, N2A cells were transfected with the indicated combinations of RNAi-inducing plasmids and rescue plasmids. The cells were lysed and analyzed by immunoblotting with anti-Chmp2b antibody. E, HeLa cells were cotransfected with HA-tagged Chmp2bWT or Chmp2bR19/22/26A together with Chmp4b-Flag. Cells were stained by dual immunofluorescence with anti-HA and anti-Flag antibodies and imaged by confocal microscopy. Insets, High magnification of the boxed regions. Scale bars, 20 μm. Colocalization of Chmp2b and Chmp4b was 80% for Chmp2bWT and 12% for Chmp2bR19/22/26A (quantified as the percentage of Chmp2b-HA-positive pixels overlapping Chmp4b-Flag puncta). F, HEK-293T cells were transfected with Flag-tagged Chmp4b plasmid, alone or together with HA-tagged Chmp2bWT or Chmp2bR19/22/26A. Anti-HA immunoprecipitates prepared from the transfected cells were analyzed by immunoblotting with anti-Flag or anti-HA antibodies. G, HEK-293T cells were transfected with Flag-tagged Chmp2bWT or Chmp2bL4D/F5D. The transfected cells were homogenized, the membrane (P) and cytosolic (S) fractions were separated by centrifugation, and equivalent amounts of each fraction were analyzed by immunoblotting with anti-Flag antibody.