Abstract
The rise in the use of biomedical devices and implants has seen a concomitant surge in the advent of device-related nosocomial (hospital-acquired) infections of bacterial and fungal origins. The most common nosocomial fungal infection is candidiasis caused mainly by Candida albicans biofilms. Candidiasis is associated with an unacceptably high mortality rate, and there is an urgent need for the discovery of new antifungal drugs that prevent or control biofilm formation. To this end, we recently developed an ultra-high-throughput microarray platform consisting of nano-scale biofilms of C. albicans encapsulated in collagen or alginate hydrogel matrices for antifungal drug screening. Here, we report that the choice of matrix influences the apparent susceptibility of C. albicans to the common anti-fungal drugs, amphotericin B and caspofungin. While amphotericin B is equally effective against biofilms grown in collagen and alginate matrices, caspofungin is effective only against biofilms grown only in alginate, but not in collagen. We demonstrate differences in the distribution of the drugs in the two matrices may contribute to the susceptibility of C. albicans nano-biofilms. In a larger context, our results highlight the importance of the choice of matrix as a parameter in 3D cell encapsulation, and suggest a screening strategy to predict drug performance in vivo.
Keywords: hydrogels, microarray, Candida albicans, biofilms, high-throughput, drug screening, caspofungin
Introduction
Biomedical devices and implants are associated with a high risk of microbial colonization and biofilm formation of surfaces, making device-related infections a serious clinical problem. A vast majority of medical-device failure is associated with nosocomial or hospital-acquired infections caused by endogenous commensals (Richards et al. 1999). C. albicans is a ubiquitous commensal found in the oral cavity and the gastrointestinal tract, which facilitates their encounter with implanted biomaterials. A wide range of biomaterials used in clinical practice support colonization and biofilm formation by Candida species, and the rise in the incidence of candida infection over the last two decades has almost paralleled the increase in the use of biomedical implants (Kojic and Darouiche 2004; Ramage et al. 2006). These device-associated biofilm infections often enter the bloodstream and disseminate to tissues. Invasive candidiasis is associated with a mortality rate as high as 40–60% (Gudlaugsson et al. 2003). One major reason for such high mortality rate is the lack of effective antifungal drugs against C. albicans biofilms.
Biofilms are structured microbial communities encased in a polysaccharide extracellular matrix. Candida forms biofilms on both biotic (such as skin and tissue) and abiotic (such as implants and catheters) surfaces. The cells in biofilm show increased resistance to most antifungal drugs and have the potential to initiate or prolong infections by providing a safe sanctuary from which organisms can invade local tissue. Clinically, Candida biofilms exhibit ~1000 fold more resistance against some of the commonly used antifungal agents compared to their planktonic counterparts (Ramage et al. 2001). In fact, it is now estimated that 60–80% of all human microbial infections involve biofilm formation (Bryers 2008; Donlan and Costerton 2002; Hall-Stoodley et al. 2004).
To address the immediate and urgent need of new antifungal drugs against Candida albicans biofilms, we have recently developed an ultra-high-throughput screening (uHTS) platform for drug discovery (Srinivasan et al. 2013; Srinivasan et al. 2011). The platform is a cellular microarray consisting of nano-scale cultures of C. albicans biofilms (‘nano-biofilms’) encased in a collagen or an alginate hydrogel matrix. These natural hydrogels have been widely used for cell-encapsulation studies because of their favorable gelation and biomimetic properties (Tibbitt and Anseth 2009). To prepare the microarray, a mixture of C. albicans yeast cells in RPMI media were mixed with collagen or alginate solution, and spotted using a robotic microarrayer on to the surface of modified glass slides. Upon incubation of the microarray for 24 h at 37 °C under humidified conditions, the yeast cells matured into biofilms. The details on optimization of culture conditions, surface modification procedure, hydrogel concentration, and characterization of the surface and biofilm growth can be found elsewhere (Srinivasan et al. 2013). The Candida albicans biofilm microarray consisted of 1200 individual spots of 30 nL volume of identical nano-biofilms encapsulated in either 1.8 mg/ml collagen or 1.5% (w/v in water) alginate. We have shown that the nano-biofilms maintained morphological, growth and phenotypic characteristics of despite a 3000-fold reduction in volume compared to the conventional 100 µl biofilms cultured in 96-well plates (Srinivasan et al. 2013).
Drug susceptibility of C. albicans nano-biofilms
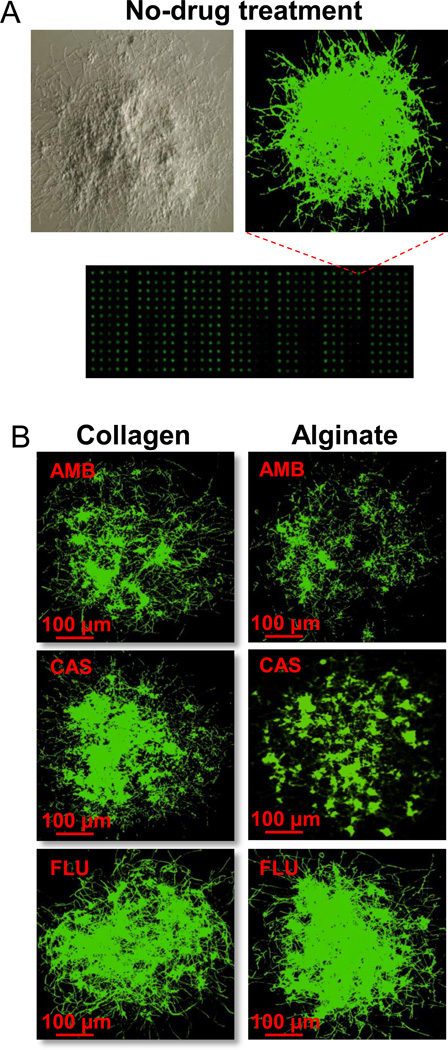
We used the nano-biofilm microarray for a direct comparison between the susceptibility of the biofilms encapsulated in collagen or alginate matrices against three common antifungal drugs — fluconazole (FLU), amphotericin B (AMB), and caspofungin (CAS). 30 nL of the drugs over a range of concentrations were printed on top of spots containing nano-biofilms. After 24h, allowing for drug action, the biofilms were stained for metabolic activity using FUN1 stain, and the fluorescence intensities were read using a microarray scanner. Using fluorescence intensity as an estimate of live cell population, the toxicity profile was prepared from normalized response wherein 100% and 0% responses correspond to entirely live and dead cells, respectively. The IC50, i.e., the inhibitory drug concentration corresponding to 50% decrease in metabolic activity compared to no drug control was calculated and is tabulated in Table 1. As expected, the nano-biofilms were not susceptible to FLU since FLU is known to be ineffective against biofilms. In fact, one of the main reasons for the resistance of biofilms against FLU, and most of the drugs from azole class, is the binding of azole molecules to glucans in the self-produced exo-polymeric ‘matrix’(Nett et al. 2007). In contrast, AMB treatment was effective against nano-biofilms encapsulated in both collagen and alginate matrices, although at relatively high concentrations that are generally considered to be toxic (Ramage et al. 2002). Interestingly, with CAS treatment, while alginate-encapsulated nano-biofilms are susceptible, the collagen-encapsulated nano-biofilms are completely resistant. In Fig. 1, we present representative scanner images of nano-biofilms encapsulated in collagen or alginate matrices, and treated with drugs at their IC50 concentrations. The green fluorescence intensity from FUN1 provides a visual description of the IC50 values.
Table 1.
A comparison of the susceptibility of C. albicans biofilms to Amphotericin B (AMB), Caspofungin (CAS) and Fluconazole (FLU) between microarray-based and 96-well plate platform.
| Platform | Nano-biofilm Microarray | |
|---|---|---|
| Drugs | Collagen IC50 (µg/mL) |
Alginate IC50 (µg/mL) |
| AMB | 0.27 ± 0.04 | 0.95 ± 0.27 |
| CAS | > 16 | 2.54 ± 0.11 |
| FLU | > 1024 | > 1024 |
Figure 1.
A microarray scanner image of Candida albicans nano-biofilms. (A) Light and fluorescence microscopic images of a mature C.albicans nano-biofilm stained with FUN1. (B) A panel of drug-treated nano-biofilms encapsulated in collagen and alginate hydrogels. The nano-biofilms were treated for 24 h with amphotericin B (AMB), caspofungin (CAS) or fluconazole (FLU) at 1 µg/ml, 3 µg/ml and 1024 µg/ml, respectively, stained with FUN1, and imaged using a microarray scanner. Filamentous hyphae can be seen attesting the presence of true biofilms.
Kinetics of drug release from hydrogels
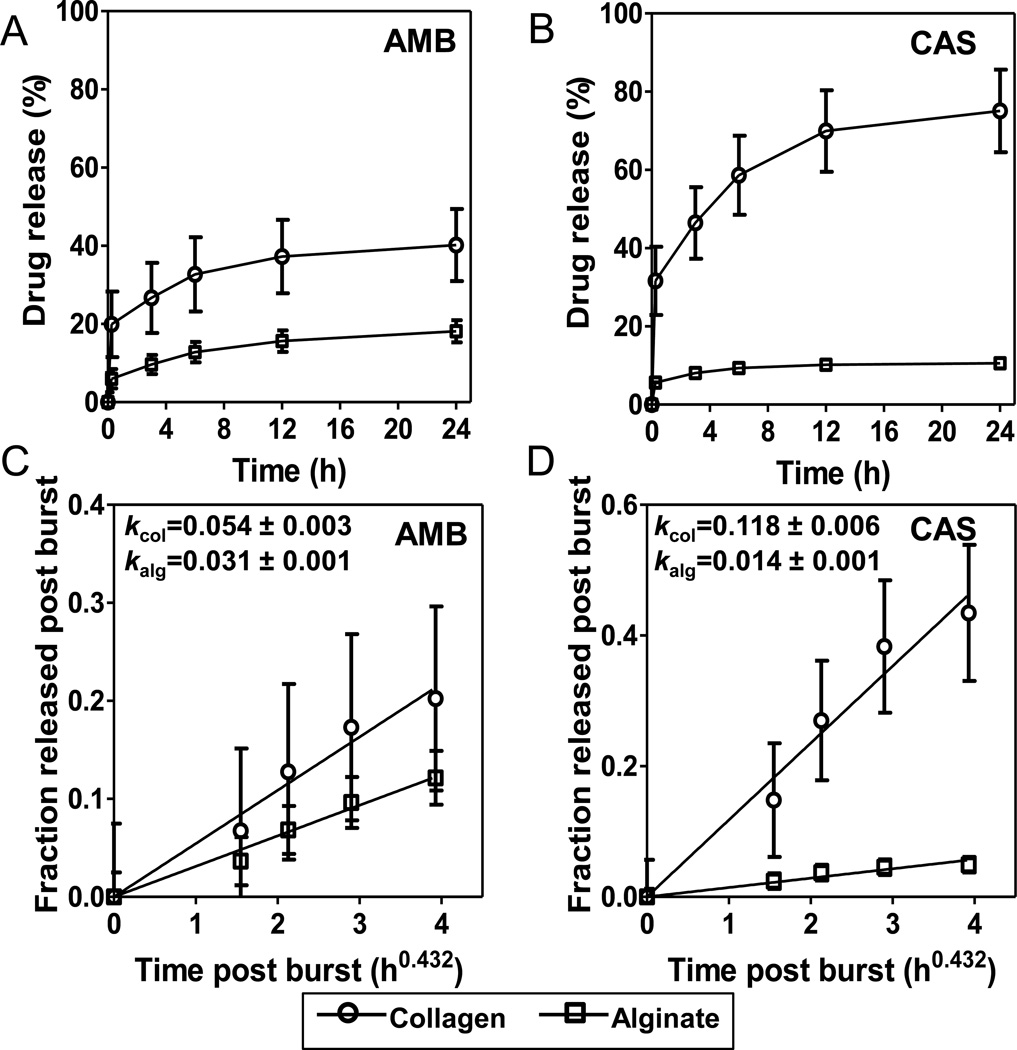
We hypothesized that the differences in susceptibilities of the nano-biofilms to AMB and CAS is because of the differences in the distribution of the drugs in collagen and alginate matrices. To test this hypothesis, we analyzed the kinetics of drug elution from the matrices by HPLC. 500 µl of collagen or alginate hydrogels were loaded with either 25 µg/ml amphotericin or 25 µg/ml caspofungin, and the elution into the solvent was measured over 24 h. The amount released was represented as a fraction of the total drug loaded into the gels (Fig. 2A and B). We observe that both drugs show a similar elution profile: a burst release within the first 15 min, followed by a slow, diffusion-limited release before attaining saturation. However, the fraction of the drug released through the assay period varied between drug–matrix combinations: for amphotericin, the burst and the subsequent diffusional releases, and the total amount released were comparable in collagen and alginate matrices (Fig. 2A); for caspofungin, the burst release was 5× more followed by a much faster diffusional release and more drug released from the collagen matrix than from the alginate matrix (Fig. 2B). This kinetic data indicates that the magnitude and the rate of release of the two drugs from collagen and alginate hydrogel matrices are dissimilar.
Figure 2.
Kinetics of release of amphotericin B (AMB), caspofungin (CAS) from collagen and alginate hydrogels. (A, B) The release of AMB and CAS from hydrogels, monitored over a period of 24 h. (C, D) Post-burst release of AMB and CAS from collagen and alginate hydrogels follows Fickian diffusion.
The burst release is characterized by the release of an initial large bolus of drug before the release rates reach a steady state. During burst release, as much as 20–90% of the drug may be released depending upon the drug-matrix system (Huang and Brazel 2001). The burst release is mostly due to non-uniform drug loading arising from stronger adsorption and desorption of the drug on the surface of the matrix than on the interior. Hence, the burst release profiles suggest that CAS is strongly adsorbed on the surface of collagen matrix but not on alginate matrix, and AMB is adsorbed somewhat more on the surface of collagen matrix than on alginate matrix. The bulk adsorption of AMB in collagen and alginate matrices results in only a fraction of total amount loaded to be released in 24 h.
The diffusional release following the initial burst is a steady state release wherein the drug diffuses out of the gel due to a concentration gradient between the gel and the solvent. For equilibrium-swollen hydrogels containing uniformly dispersed drugs, the Fickian diffusional release from spheres is given by the following semi-empirical relationship (Ritger and Peppas 1987):
where and k are fraction of drug released in time t and slope parameter, respectively. k is a structural and geometric parameter that is related to the effective diffusivity of drug in the hydrogel D and thickness of the matrix δ over which the drug is distributed by: k~(D/πδ2)0.43. Thus, k is a lumped parameter that accounts for several factors including properties of the drug, matrix or their interactions, which is implicit in D and δ. To analyze only the diffusional release, the post burst data from Fig. 2A and B was fitted to the above equation. As shown in Fig. 2C and D, the data fit well to a linear relationship between and t0.43 (r2 > 0.95), suggesting that drug release occurs by Fickian diffusion. The estimated values of k are also listed in the Fig. 2C and D. Since k value for AMB in collagen (0.054) is only marginally (1.7×, P=0.01) different from that in alginate (0.031), the diffusional transport rates of AMB is only slightly larger in collagen than in alginate hydrogels. In contrast, the k value for CAS in collagen (0.118) is considerably larger (8.4×, P=0.0001) than alginate (0.014) implying that diffusional transport rate of CAS in collagen is much higher than in alginate.
Since k = k(D, δ), the difference in k values for AMB and CAS between collagen and alginate hydrogels can either be due to differences in the diffusivity of the drug D or the distribution, i.e., the thickness of the matrix δ over which the drug distributed. Both AMB and CAS are uncharged, hydrophilic small molecules with molecular weights of 0.92 and 1.1 kDa, and estimated octanol/water partition coefficient, log P (o/w) of −2.8 and −3.8, respectively. The hydrodynamic radii of these molecules are estimated to be 0.6–0.7 nm for a spherical geometry. The effective diffusivity of the drugs through the macroporous gel can be estimated from (Ogston et al. 1973): , where De and D0 are effective diffusivity in hydrogel and free diffusivity, respectively; rs and rg are hydrodynamic radii of the drug and alginate/collagen fibers in the gel, respectively; and ϕ is the volume fraction of the polymer (alginate/collagen) in the hydrogel. Since collagen forms long fibers, rg ~200 nm, and ϕ ~0.01, the Ogston equation yields De ~0.9D0 (Mu et al. 2007) Since alginate forms gel only by crosslinking, rg ~0.6–0.8 nm, and ϕ ~0.01, the Ogston equation yields the De ~0.8D0 (Amsden 1998). Further, the hydrogel matrices contain >90% water with pore sizes ranging between 1–3 µm for collagen gel (Mickel et al. 2008) and 10–100 nm for alginate gel (Leal-Egana et al. 2011) indicating that alginate has smaller pores for drug transport. It has been reported that the tortuosity due to the network structure of collagen and alginate hydrogels decreases diffusivity of molecules of ~1 kDa only modestly, i.e., D in the hydrogel is 70–90% of that of free diffusion in water (Chan and Neufeld 2010; Li et al. 1996; Ramanujan et al. 2002). Taken together, these estimates mean that the alginate matrix may offer somewhat more resistance to drug diffusion than the collagen matrix. Therefore, the large difference in k values for CAS between collagen and alginate gels is likely more due to the difference in the thickness δ over which the drug is distributed. Since k ~1/δ, larger k for CAS in collagen means the drug is distributed over a thin shell along the periphery of the gel resulting in faster release. Conversely, a lower value of k for alginate implies that CAS is distributed over a thicker zone, or even possibly, throughout the gel. Similarly, the small difference in k values for AMB between the alginate and collagen matrices implies a similar drug distribution between the gels.
Drug distribution in hydrogels
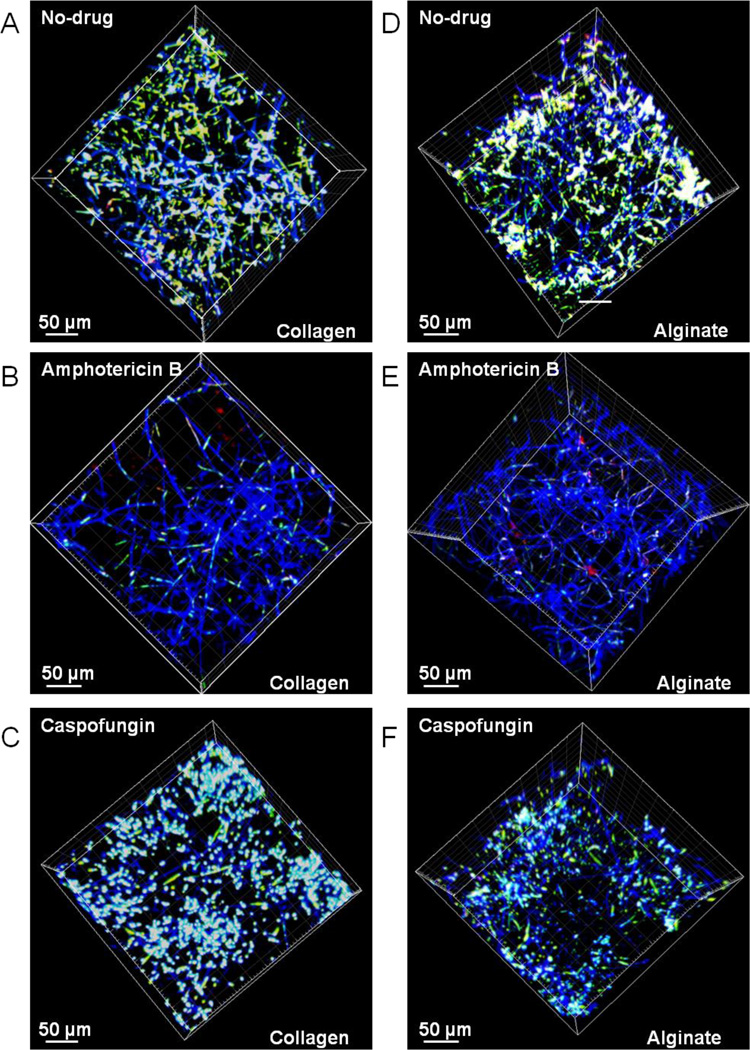
To experimentally validate the aforementioned argument, we tested the penetration of AMB and CAS into collagen and alginate hydrogels using cell viability as a surrogate measure. Nano-biofilms grown in collagen and alginate hydrogels were exposed to 4 µg/ml of CAS and AMB for 24 h. Nano-biofilms exposed to no drugs were used as control. The biofilms were stained with Calcofluor White for cell wall (blue fluorescence), and FUN1 for cell viability (red/green fluorescence), and analyzed by confocal laser scanning microscopy (CLSM). From the 3D microscopic images shown in Fig. 3, several observations can be made: (i) the nano-biofilm growth in 3D is similar in the two matrices in the absence of any drugs (Fig. 3A and B). The cell wall along the hyphae appears as continuous blue filaments stained by Calcofluor White, and the vacuoles in live cells as bright white spots due to a combination of red/green fluorescence from FUN1 metabolism superimposed on a blue background; (ii) treatment with AMB decreases the viability of nano-biofilms throughout the collagen and alginate matrices (Fig. 3B and E). The cell wall of dead cells fluoresce blue without any red/green fluorescence from metabolically active cells; and (iii) treatment with CAS decreases the viability of nano-biofilms in alginate but not in collagen matrices as seen by the presence of active vacuoles as white spots in the latter but not in the former (Fig. 3C and F). Further, the depth perspective of these images qualitatively indicates a spatial variation in cell viability particularly for CAS treatment.
Figure 3.
Confocal Laser Scanning Microscopy (CLSM) images of nano-biofilms encapsulated in collagen (A,B,C) and alginate (D,E,F) matrices after staining for metabolically active cells with FUN1 and cell wall with Calcofuor White M2R. The biofilms demonstrate a 3D architecture with cell wall from hyphae (blue) superimposed with vacuoles of live cells (yellow). The viability of cells not exposed to any drugs (A, D) decreases after the cells were exposed to either AMB (B, E) or CAS (C, F). Further, the biofilms encapsulated in collagen and alginate gels are equally susceptible when treated with AMB (B, E) but the biofilms encapsulated in collagen gels (C) are more viable than in alginate gels (F) when treated with CAS.
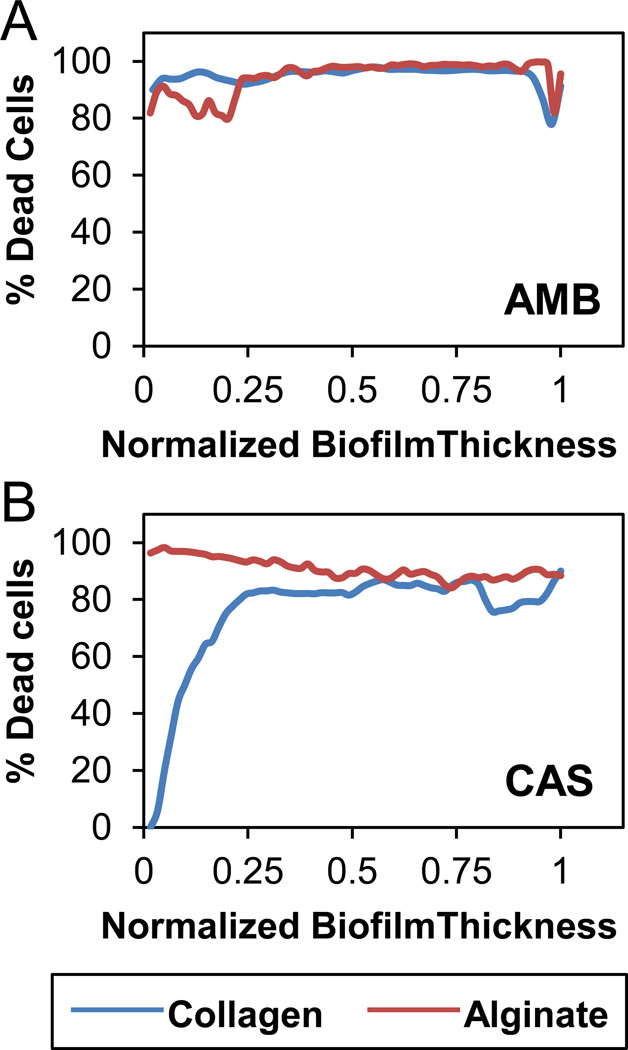
To quantify the distribution of live cells through the depth of the biofilm, we horizontally sectioned the 3D confocal microscopic images along the vertical Z-axis into 2D X-Y slices, and estimated the fraction of metabolically active cells. The cell viability distribution for no drug, AMB and CAS treatments in nano-biofilms grown in collagen and alginate matrices are shown in Fig. 4. As expected, all cells were viable throughout the biofilm when not treated with drugs. While AMB treatment resulted in cell death throughout the biofilm (Fig. 4A), CAS treatment showed differences in the distribution of viable cells between biofilms encapsulated in alginate and collagen matrices (Fig. 4B). In alginate gels, the cell viability was low throughout the biofilm but in collagen gels, the cell viability was low only at the top of the biofilm but was 100% at the bottom. This result supports our hypothesis that CAS is strongly adsorbed on the periphery of the collagen matrix, and hence may not reach the cells at the bottom of the biofilms. These analyses, together with release data from Fig. 2, suggest that AMB is distributed throughout the collagen and alginate matrices and hence is released slowly while CAS is distributed throughout alginate matrix and is released slowly but is distributed only on the periphery of collagen matrix and hence is released rapidly.
Figure 4.
Cell viability along the depth of 3D nano-biofilms. The 3D CLSM images of nano-biofilms were horizontally sectioned and the viability of cells was calculated at each section from the top (1) to bottom (0). (A) AMB treatment kills cells uniformly at all sections of the biofilms encapsulated in collagen or alginate gels; (B) CAS treatment kills cells at all sections only when the biofilms were encapsulated in alginate gel but not in collagen gel since a majority of cells at the bottom of the biofilm are viable and hence unexposed to the drug.
Significance
The apparent inactivity of CAS against collagen-encapsulated biofilms vs. activity against alginate-encapsulated biofilms in the microarray resembles an increase in IC50 of CAS by up to 16× against various strains of C. albicans biofilms in the presence of 50% serum compared to 0% serum in 96-well plate assays (Odabasi et al. 2007). A similar or even higher increase in IC50 in the presence of serum has been reported for other echinocandin drugs, micafungin and anidulafungin (Paderu et al. 2007). This increase is because CAS and other echinocandin drugs bind extensively (95%) to serum proteins, which impairs their ability to inhibit glucan synthase and hence restrict fungal wall biosynthesis (Eschenauer et al. 2007; Paderu et al. 2007). As a result, the prediction of in vivo efficacies of CAS following systemic infusion based on standard 96-well plate in vitro IC50 has been unreliable (Lazzell et al. 2009). In such situations, it appears that the antifungal susceptibility tests of biofilms encapsulated in collagen hydrogels may better mimic the in vivo response during invasive forms of the disease.
In summary, we have demonstrated the Candida albicans biofilms encapsulated in an alginate or a collagen matrix exhibit differential susceptibility to the common antifungal drugs, amphotericin B and caspofungin due to the interaction or lack thereof between the matrices and the drugs. The impact of the results presented in this work is not restricted to antifungal drug screening but broadly applicable to other 3D-cell based screens (Lee et al. 2008). 3D cultures of mammalian cells encapsulated in natural or synthetic polymeric matrices are touted as a more faithful replica of in vivo tissue architecture than 2D monolayer cultures (Meli et al. 2012; Tibbitt and Anseth 2009). The natural matrices widely used for cytotoxicity assays are collagen or matrigel, which have the advantage of reproducing extracellular matrix environment or alginate, which by virtue of being inert is an excellent system for isolating cellular responses (Smidsrod and Skjak-Braek 1990). We propose that screening for activity in these two matrices, i.e., an inert carbohydrate matrix such as alginate and a protein matrix such as collagen, may be a useful assay strategy to evaluate the effect of the drug on cells per se in isolation on one hand vs. the response of the cells in an extracellular matrix environment in vivo on the other.
Materials and Methods
Nano-biofilm culture and drug susceptibility assays
C. albicans SC5314, a biofilm forming strain, was propagated overnight in 20 mL of Yeast Peptone Dextrose (YPD) media at 30 °C. Cells harvested were washed in PBS and adjusted to a seeding density of 5×106 cells/mL in RPMI. To this cell suspension,1.8 mg/mL collagen or 1.5% (w/v in water) alginate was added, and 50 nL of this solution was printed by non-contact deposition using a robotic microarrayer (Microsys, Digilab Inc., Marlborough, MA) on to PS-MA-modified glass slides (Lee et al. 2008; Srinivasan et al. 2012). The media, cell seeding density, PSMA coating solution and collagen/alginate concentrations were optimized to yield robustly attached, hemispherical spots (Srinivasan et al. 2012). The slides were then placed in a hybridization cassette (Array It, Sunnyvale, CA) to prevent evaporation and incubated at 37°C to allow biofilm formation. The susceptibility profile of C. albicans biofilms against clinically used antifungals agents, fluconazole, amphotericin B and caspofungin was obtained by printing equal volumes of desired concentrations of drugs on top of the biofilms. After 24 h at 37°C, allowing for drug action; the biofilm array was stained with 0.5 µM FUN1 and the metabolic activity of cells within the biofilms were read using a microarray scanner (GenePix 4100A, Molecular devices, Sunnyvale, CA). Thus, depending upon the efficacy of the drug and its dose, each spot exhibits different fluorescence intensity. The susceptibility profile of each drug was determined based on the reduction in fluorescent intensity quantified in comparison with the positive (no-drug) and negative control (no-cell). For Confocal Laser Scanning Microscopy (CLSM), the biofilms were stained with 15 µM FUN1 and 20 µM Calcofluor White M2R (Life Technologies, Grand Island, NY) and imaged using an LSM 510 (Zeiss) with 40x water immersion lens. The fraction of metabolically active cells through the depth of the biofilm was estimated from the ratio of FUN1 fluorescence intensity from live cells to Calcofluor White intensity from cell walls in each slice of the 3D image (AutoQuant X2, Media Cybernetics, Rockville, MD).
Kinetics of drug elution from hydrogels
The kinetics of elution of antifungal drugs from hydrogel matrices was studied using High Performance Liquid Chromatography (HPLC) (Agilent Technologies, Santa Clara, CA). Briefly, 500 µL of 1.8 mg/mL collagen hydrogel was cast in chamber slides and incubated at 37°C for 30 min for gelation. To cast alginate hydrogels, the chamber slides were pretreated with 200 µL of 0.1N BaCl2 and 500 µL of 1.5% alginate was added. After gelation, the collagen and alginate hydrogels were loaded with equal volume of 50 µg/mL amphotericin B or 50 µg/mL of caspofungin. After 24 h of drug-sorption, the hydrogels were immersed in 1mL of HPLC grade water. Using Poroshell 120 C18 column (4.6 mm × 50mm, 2.7 µm), the release of antifungal was monitored isocratically over 24 h. The details of HPLC experimental parameters are tabulated in Table S1.
Statistics
The drug susceptibility experiments were performed in duplicate microarrays with 6–8 replicates at each drug concentration per microarray. The drug elution experiments were performed in quadruplicate. The data was analyzed using Prism (GraphPad, La Jolla, CA) and the results were considered significant if P<0.05 by one-tailed Student’s t-test.
Supplementary Material
Acknowledgements
This work was funded in part by the US Army Medical Research and Materiel Command, Combat Casualty Care Research Directorate intramural funding program; biofilm-related work in the J.L.L.-R. laboratory is funded by NIH (1R01DE023510-01) and by DOD (W911NF-11-1-0136), and infectious diseases-related work in the A.K.R. laboratory is funded by NIH (SC1HL112629). Confocal microscopy was performed at the RCMI Advance Imaging Center, supported by the NIH (5G12 RR01 3646-10). The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the DOD or the NIH. We also would like to thank an anonymous reviewer for significant improvement in our interpretation of the results.
Reference
- Amsden B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules. 1998;31:8382–8395. [Google Scholar]
- Bryers JD. Medical biofilms. Biotechnol Bioeng. 2008;100(1):1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Neufeld RJ. Tunable semi-synthetic network alginate for adsorptive encapsulation and controlled release of protein therapeutics. Biomaterials. 2010;31(34):9040–9047. doi: 10.1016/j.biomaterials.2010.07.111. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenauer G, Depestel DD, Carver PL. Comparison of echinocandin antifungals. Ther Clin Risk Manag. 2007;3(1):71–97. doi: 10.2147/tcrm.2007.3.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37(9):1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17(2):255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzell AL, Chaturvedi AK, Pierce CG, Prasad D, Uppuluri P, Lopez-Ribot JL. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. Journal of Antimicrobial Chemotherapy. 2009;64(3):567–570. doi: 10.1093/jac/dkp242. [DOI] [PubMed] [Google Scholar]
- Leal-Egana A, Braumann UD, Diaz-Cuenca A, Nowicki M, Bader A. Determination of pore size distribution at the cell-hydrogel interface. J Nanobiotechnology. 2011;9:24. doi: 10.1186/1477-3155-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Kumar RA, Sukumaran SM, Hogg MG, Clark DS, Dordick JS. Three-dimensional cellular microarray for high-throughput toxicology assays. Proc Natl Acad Sci U S A. 2008;105(1):59–63. doi: 10.1073/pnas.0708756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RH, Altreuter DH, Gentile FT. Transport characterization of hydrogel matrices for cell encapsulation. Biotechnol Bioeng. 1996;50:365–373. doi: 10.1002/(SICI)1097-0290(19960520)50:4<365::AID-BIT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Meli L, Jordan ET, Clark DS, Linhardt RJ, Dordick JS. Influence of a three-dimensional, microarray environment on human cell culture in drug screening systems. Biomaterials. 2012;33:9087–9096. doi: 10.1016/j.biomaterials.2012.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel W, Munster S, Jawerth LM, Vader DA, Weitz DA, Sheppard AP, Mecke K, Fabry B, Schroder-Turk GE. Robust pore size analysis of filamentous networks from three-dimensional confocal microscopy. Biophys J. 2008;95(12):6072–6080. doi: 10.1529/biophysj.108.135939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu C, Li D, Lin W, Ding Y, Zhang G. Temperature induced denaturation of collagen in acidic solution. Biopolymers. 2007;86(4):282–287. doi: 10.1002/bip.20742. [DOI] [PubMed] [Google Scholar]
- Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, VanHandel M, Andes D. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51(2):510–520. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odabasi Z, Paetznickz V, Rex JH, Ostrosky-Zeichner L. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob Agents Chemother. 2007;51(11):4214–4216. doi: 10.1128/AAC.01589-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston AG, Preston BN, Wells JD. On the transport of compact particles through solutions of chain-polymers. Proc R Soc Lond A. 1973;333:297–316. [Google Scholar]
- Paderu P, Garcia-Effron G, Balashov S, Delmas G, Park S, Perlin DS. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob Agents Chemother. 2007;51(6):2253–2256. doi: 10.1128/AAC.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006;6(7):979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45(9):2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, VandeWalle K, Bachmann SP, Wickes BL, Lopez-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002;46(11):3634–3636. doi: 10.1128/AAC.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys J. 2002;83(3):1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27(5):887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. Journal of Controlled Release. 1987;5(1):23–36. [PubMed] [Google Scholar]
- Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8(3):71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Leung KP, Lopez-Ribot JL, Ramasubramanian AK. High-throughput nano-biofilm microarray for antifungal drug discovery. mBio. 2013;4(4) doi: 10.1128/mBio.00331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, Lopez-Ribot JL, Ramasubramanian AK. Candida albicans biofilm chip (CaBChip) for high-throughput antifungal drug screening. J Vis Exp. 2012;(65):e3845. doi: 10.3791/3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, Uppuluri P, Lopez-Ribot J, Ramasubramanian AK. Development of a high-throughput Candida albicans biofilm chip. PLoS ONE. 2011;6(4):e19036. doi: 10.1371/journal.pone.0019036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnology and Bioengineering. 2009;103(4):655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.