Fig. 1.
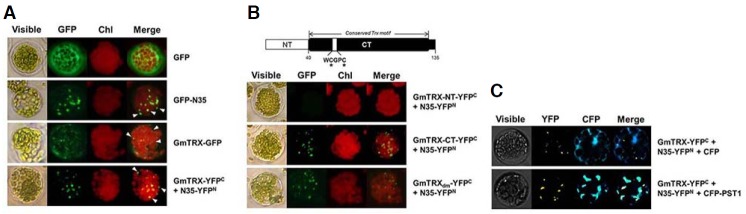
Analysis of the interaction between GmTRX and nodulin-35 (N35) in Arabidopsis protoplasts. Bimolecular fluorescence complementation (BiFC) analyses of the interaction between GmTRX and N35 were performed as follows: expression of P35S-GFP as a control (GFP; A); expression of N35 fused to GFP (GFP-N35; A); expression of GmTRX fused to GFP (GmTRX-GFP; A); coexpression of P35S-GmTRX-YFPC and P35S-N35-YFPN (GmTRX-YFPC + N35-YFPN; A); coexpression of P35S-GmTRX-NT-YFPC and P35S-N35-YFPN (GmTRX-NT-YFPC + N35-YFPN; B); coexpression of P35S-GmTRX-CT-YFPC and P35S-N35-YFPN (GmTRX-CT-YFPC + N35-YFPN; B); coexpression of P35S-GmTRXdm-YFPC and P35S-N35-YFPN (GmTRXdm-YFPC + N35-YFPN; B); coexpression of P35S-GmTRX-YFPC, P35S-N35-YFPN and P35S-CFP (GmTRX-YFPC + N35-YFPN + CFP; C); and coexpression of P35S-GmTRX-YFPC, P35S-N35-YFPN and P35S-CFP-PTS1 (GmTRX-YFPC + N35-YFPN + CFP-PTS1; C). White arrowheads in (A) indicate the peroxisome-like area. A schematic representation of the domain structure of GmTRX used in the BiFC analysis is shown in (B); NT, the region of amino acids from No. 1 to 40; CT, the rest of the whole protein. Conserved cysteines in the catalytic domain (C59 and C62) are indicated. GmTRXdm: a mutated form of GmTRX with catalytic cysteins substituted (see “Materials and Methods”). PTS1: peroxisomal targeting signal 1. Chl: autofluorescence of chloroplasts. These experiments were replicated three times with similar results.
