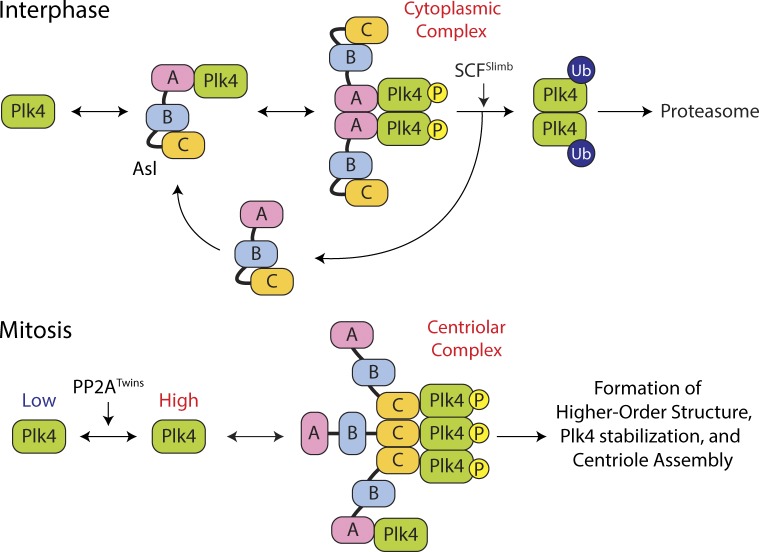
Figure 7.
Model of Plk4 turnover regulation by domain-specific and cell cycle–dependent activities of Asl. (top) A cytoplasmic Asl–Plk4 complex forms during interphase to facilitate Plk4 degradation. Asl-A binding to Plk4 is dominant and facilitates Plk4 homodimerization and autophosphorylation, triggering its ubiquitination by SCFSlimb and degradation. (bottom) A centriolar Asl–Plk4 complex forms during mitosis to stabilize Plk4 and initiate centriole duplication. PP2ATwins counteracts Plk4 autophosphorylation, allowing Plk4 protein levels to rise. Asl-C trimers bind Plk4 dimers, forming high-order structures that protect Plk4 from proteasome-mediated degradation. Asl-A also participates in stabilizing mitotic Plk4, but the mechanism by which this occurs is unclear. Plk4 aggregates into an asymmetric spot on the centriole surface, which modifies the centriole, making it competent to assemble a single daughter during S phase. P, phosphorylation.