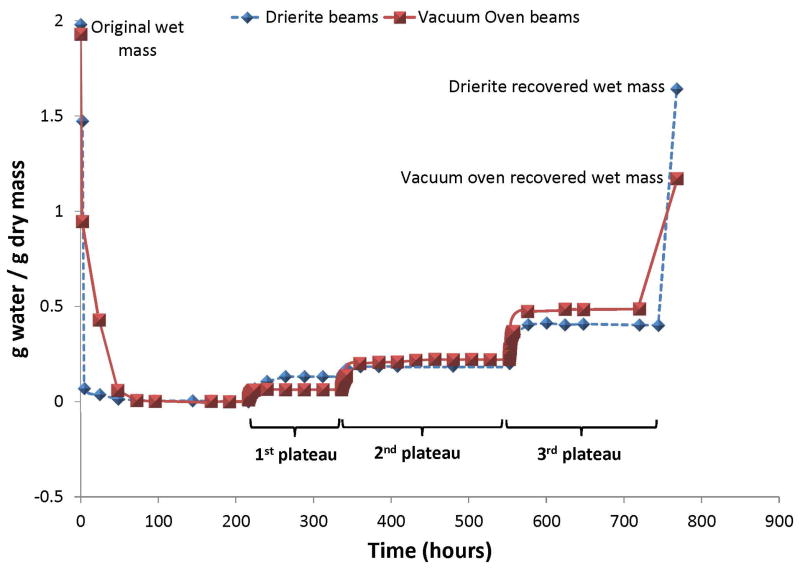
Fig. 2.
Change in water content (g H2O/g dry mass) of absolutely dry demineralized dentin beams over time. The specimens that were dried for 2 hrs in the vacuum oven had a water content of 0.946 g H2O/g dry mass (squares). This fell to 0.429 and then to 0.059 g H2O/g dry mass in the vacuum oven at 37°C over the next 50 hrs. Then the oven temperature was increased 10°/hr until the water content fell to 0.001 g H2O/g dry mass. Specimens dried in Drierite for 2 hrs had a water content of 1.474 g H2O/g dry mass that fell to 0.001 g H2O/g dry mass after 200 hrs in 0% RH Drierite. Then both groups (n = 10) were exposed to 41% RH and weighed until they reached their first plateau in mass gain, at a water content of 0.064 g H2O/g dry mass (vacuum oven group, squares) or 1.116 g H2O/g dry mass (Drierite group, diamonds). The beams were then exposed to 60% RH water vapor and reweighed until they reached their second plateau in mass at 0.220 g H2O/g dry mass (squares) or 0.191 g H2O/g dry mass. They were then placed in water vapor of 99% RH until they reached their third plateau in mass at 0.401 g H2O/g dry mass for the Drierite group (diamonds) or 0.493 g H2O/g dry mass for the vacuum oven group (squares). Both groups were then immersed in liquid water at 25°C for 1 hr to fully hydrate. The final water content was 1.71 g H2O/g dry mass for the Drierite group (diamonds) or 1.71 g H2O/g dry mass for the vacuum oven group (squares), showing that the vacuum group did not fully recover their initial hydration.