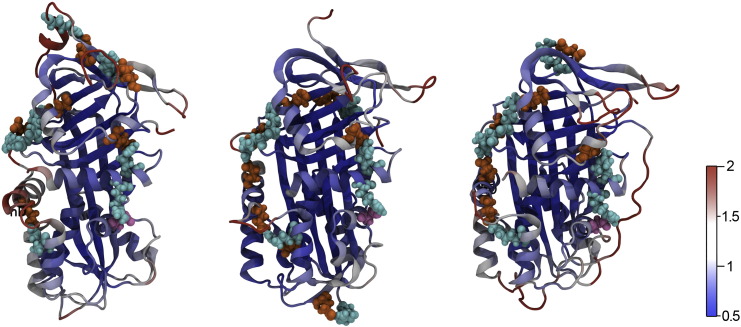
Fig. 5.
RMSF of the NS conformers. Cα RMSF mapped onto the average NS structure colored according to displacement during 45 ns, for the three NS conformers (with helix F on the back as in the right panel of Fig. 1): native (left), cleaved (center), latent (right). The structural elements are color-coded from blue (RMSF: 0.5 Å, stable) to red (RMSF: 2.0 Å, flexible), as in the color scale bar on the right. Note the hD helix flexibility, which is higher in the native than in cleaved and latent forms. The most stable salt bridges are represented as colored spheres: arginine (orange), glutamate (cyan), and aspartate (pink).