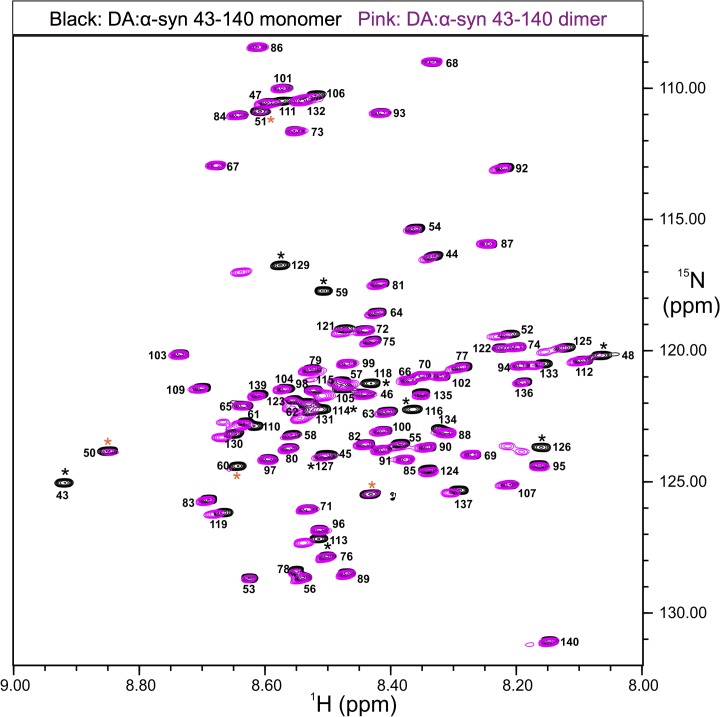
Fig 6. Comparison of 1H, 15N-HSQC spectra acquired on DA:α-syn 43–140 monomer and α-DA:syn 43–140 dimer showing the characteristics expected for an intrinsically disorder protein.
The proton-nitrogen correlation spectrum (HSQC) of 0.5 mM DA:α-syn 43–140 monomer or DA:α-syn 43–140 dimer in 10 mM sodium phosphate buffer, pH5.5. Spectra were acquired at 5°C and 800MHz. The HSQC spectrum of DA:α-syn 43–140 monomer is depicted in black and the DA:α-syn 43–140 dimer in pink. Amide resonance assignments are indicated for the DA:α-syn 43–140 monomer as sequence position and the poor chemical shift dispersion indicates little if any secondary structure is present. * indicate residues of interest, those colored orange indicate resonances with exchange broadening (see the text).