Abstract
Malignant forms of glioma, the most common primary brain tumors, remain poorly responsive to multimodality therapeutic interventions, including chemotherapy. Suppressed apoptosis and extraordinary invasiveness are important distinctive features that contribute to the malignant phenotype of glioma. We have developed the vascular endothelial growth factor receptor 1 (VEGFR-1/flt-1) conditional replicating adenoviral vector (CRAdRGDflt-IL24) encoding the interleukin-24 (IL-24) gene. We investigated whether a combination of CRAdRGDflt-IL24-mediated oncolytic virotherapy and chemotherapy using temozolomide (TMZ) produces increased cytotoxicity against human glioma cells in comparison with these agents alone. Combination of CRAdRGDflt-IL24 and TMZ significantly enhanced cytotoxicity in vitro, inhibited D54MG tumor growth and prolonged survival of mice harboring intracranial human glioma xenografts in comparison with CRAdRGDflt-IL24 or TMZ alone. These data indicate that combined treatment with CRAdRGDflt-IL24-mediated oncolytic virotherapy and TMZ chemotherapy provides a promising approach for glioma therapy.
Keywords: IL-24, glioma, CRAd
Introduction
Gliomas are the most common primary adult brain tumors and one of the most difficult to treat malignant diseases. The unprecedented resistance of malignant gliomas to radiation therapy and chemotherapy because of their highly invasive nature and remarkable heterogeneity that reflects the genomic instability of these tumor cells contribute substantially to the fact that patient median survival has not appreciably changed in the last several decades despite highly aggressive therapeutic approaches. This cancer poses an extraordinary challenge to develop novel, safe and effective therapies that can be integrated into the traditional therapeutic tripod of surgery, radiotherapy and chemotherapy. This aim has been pursued by several therapeutic strategies including gene therapy, oncolytic virotherapy, vaccination and adoptive immunotherapy.
A growing body of evidence demonstrates the promise of gene therapy and oncolytic virotherapy in preclinical and early clinical studies. Gene therapy techniques to the brain have been generally based on the direct injection of the gene transfer vector into the tumor mass or into the surgical cavity margin after tumor debulking. Unfortunately, results of several clinical trials are rather disappointing in terms of clinical response, even though treatment procedures and gene transfer have been generally proven to be safe and well tolerated.1,2 The reasons for such failure have been mainly linked to the low efficiency of transduction of target tumor cells (as reported for the majority types of cancers), rather than to the gene therapy strategy itself. Also, gene therapy eventually may increase the therapeutic response in combination with conventional therapies and lead to an expansion of the multimodality approach that is the current standard of care.
Considering the extraordinary invasiveness of gliomas, this type of cancer presents very special difficulties in translating a positive in vitro experiment into an effective patient therapy. Malignant gliomas are known to invade surrounding normal brain tissue, which results in incomplete surgical resection and high incidence of local recurrence. Brain tumor growth and metastasis are accompanied by formation of new blood vessels. Data from experimental and clinical studies indicate that glioma is a strongly angiogenesis-dependent cancer.3,4 The overexpression of the vascular endothelial growth factor (VEGF) in cancer cells promotes tumor angiogenesis by activation of the VEGF receptor expression in tumor vessels compared with surrounding normal endothelial cells.5–8 Co-expression of VEGF and its receptors by brain tumor cells raises the possibility of autocrine stimulation of malignant glioma development and dissemination as well as therapeutic strategies targeting these receptors.9–12 Thus, vascular-targeted anticancer therapy is an attractive strategy for glioma.
Induction of apoptosis in tumor cells is an important cytotoxicity mechanism of most anticancer therapies. However, inhibition of apoptosis is an essential distinctive feature that contributes to the malignant phenotype of glioma. Thus, due to the intrinsic resistance of gliomas to chemotherapy, current attempts to improve the survival of patients largely depend on strategies to target apoptosis pathways.13,14
To address these issues, we have developed a retargeted conditional replicating adenoviral (CRAd) vector (CRAdRGDflt-IL24) employing the VEGFR-1/flt-1 promoter to control E1a gene expression with an RGD peptide inserted into the HI-loop of the Ad fiber knob domain and encoding the interleukin-24 (IL-24) gene. The rationale behind the design of this vector is based on the following observations: (1) the utilization of retargeted CRAd by expression of RGD in the fiber knob improves vector transduction;15,16 (2) the employment of the VEGFR-1/flt-1 promoter restricts CRAd replication to cancer cells and tumor-associated microvessels overexpressing VEGF;17–19 and (3) IL-24 selectively induces apoptosis in malignant cells and inhibits angiogenic activity.20–22 We investigated whether a combination of CRAdRGDflt-IL24-mediated oncolytic virotherapy and chemotherapy using temozolomide (TMZ) produces increased cytotoxicity against human glioma cells in comparison with these agents alone. Combination of CRAdRGDflt-IL24 and TMZ significantly enhanced cytotoxicity in vitro, inhibited tumor growth and prolonged survival of mice harboring intracranial human glioma xenografts in comparison with these treatments alone. The application of such strategies of combination chemotherapy and oncolytic virotherapy with dual action employing apoptosis-inducing and angiogenesis-suppressing IL-24 expression, offers unique opportunities to develop a more effective glioma treatment regimen.
Materials and methods
Cells and reagents
Human glioma cell lines D54MG, U251MG and U373MG (a kind gift from Dr D Bigner, Duke University, Durham, NC) and human embryonic kidney cells HEK293 (Microbix Biosystems, Ontario, Canada) were cultured in DMEM/F12 (Mediatech, Herndon, VA) containing 10% fetal bovine serum (FBS) (Summit Biotechnology, Fort Collins, CO). The human umbilical vein endothelial cells HUVEC were purchased from Clonetics (Walkersville, MD) and were grown in EGM-2 growth media (Clonetics). The human normal bronchial epithelial cells BEAS-2B (American Type Culture Collection, Manassas, VA) were grown in BEGM growth media (Clonetics). All cells were cultured at 37 °C in a humidified atmosphere with 5% CO2 atmosphere without antibiotics.
Temodar (temozolomide, TMZ) was obtained from Schering Corporation (Kenilworth, NJ), recombinant human IL-24 from R&D Systems (Minneapolis, MN) and recombinant human VEGF was purchased from Endogen (Wobum, MA).
Adenoviral vectors
Conditional replicating adenoviral (CRAd) vectors were constructed using standard molecular cloning methods. Briefly, polymerase chain reaction (PCR) was employed to generate a PCR product encoding a fragment of the E1 gene (nucleotides 560–3533) using pXC1 plasmid (Microbix Biosystems) as template. The PCR product was cloned into a plasmid pShuttle (Quantum Biotechnologies, Montreal, Canada) using XhoI and SalI sites to generate the pShuttleE1. VEGFR-1 promoter element was cleaved from pShuttle-flt-Luc plasmid and ligated with XhoI-digested and blunted using DNA Polymerase I Klenow fragment pShuttleE1 plasmid to produce the pShuttle-flt-E1. To develop the pShuttle-flt-E1-IL24 plasmid encoding the E1a gene under control of the human VEGFR-1 promoter element and interleukin-24 (IL-24/mda-7) gene, the fragment including the IL-24 open reading frame and SV40 late polyadenylation signal under control of the eEF-1A-HTLV hybrid promoter was replaced from pORF9-hIL24 plasmid (InvivoGen, San Diego, CA) using SgfI and SwaI sites, then blunted using DNA Polymerase I Klenow fragment and cloned into a pShuttle-flt-E1 plasmid. The inserts were confirmed by using restriction enzyme mapping and partial sequencing analysis.
Recombinant Ad genomes were generated by homologous DNA recombination in E. coli BJ5183 between pShuttle-flt-E1-IL-24 and pVK503C plasmids, which were kindly provided by Dr V Krasnykh (MD Anderson Cancer Center, Houston, TX), containing a gene encoding CDCGRDCFC peptide inserted into the HI-loop of the Ad fiber knob protein (CRAdRGDflt-IL24), or between pShuttle-flt-E1 and pAdEasy-1 (Quantum Biotechnologies) or pVK503C plasmids, to generate CRAdflt or CRAdRGDflt, respectively. The newly generated genomes were confirmed by partial sequencing analysis, linearized with PacI and transected into HEK293 cells using the SuperFect Transfection Reagent (QIAGEN, Chatsworth, CA) to generate CRAdRGDflt-IL24, CRAdflt and CRAdRGDflt recombinant Ads. Adflt-Luc (encoding firefly luciferase (Luc) gene under control of the VEGFR-1 promoter) and AdCMV-Luc (encoding Luc under control of the CMV promoter) were kindly provided by Dr P Reynolds (University of Adelaide, Adelaide, Australia). All viruses were propagated in HEK293 cells, purified by cesium chloride gradient ultracentrifugation and subjected to dialysis. Viral titer was measured by a 50% tissue culture infectious dose (TCID50) assay using the Karber equation: , where T is infectious titer in TCID50/ml, D is the log10 of the dilution, S is the log10 for the initial dilution plus the sum of ratios and V is the volume in ml of the diluted virus used for infection. Also, viral titer was measured by absorbance of the dissociated virus at A260 nm. Multiplicity of infection for subsequent experiments was expressed as TCID50 per cell.
RNA preparation and RT-PCR
The levels of VEGFR-1 and IL-24 receptor mRNA were determined by reverse transcriptase PCR (RT-PCR). Total RNA was extracted from 1 × 107 cells using RNeasy Mini Kit (Qiagen, Valencia, CA), following standard protocol, and quantified spectrophotometrically using an MBA 2000 spectrophotometer (Perkin Elmer, Wellesley, MA). cDNA was synthesized using random hexamer primers and an Omniscript RT kit (Qiagen). The first-strand cDNA was used as the template for PCR. For amplification of cDNA encoding VEGFR-1, IL-20R1, IL-20R2 and IL-22R2 the following primers were used 5′-GAGGATTCTGACGGTTTC-3′ (Flt-1 forward); 5′-CCTGTCAGTATGGCATTG-3′ (Flt-1 reverse); 5′-TCAAACAGAACGTGGTCCCAGTG-3′ (IL-20R1 forward); 5′-TCCGAGATATTGAGGGTGATAAAG-3′ (IL-20R1 reverse); 5′-GCTGGTGCTCACTCACTGAAGGT-3′ (IL-20R2 forward); 5′-TCTGTCTGGCTGAAGGCGCT GTA-3′ (IL-20R2 reverse); 5′-ACACGGTCTACAGCATCGAGTATAA-3′ (IL-22R1 forward); 5′-CGGTGACCCTGGCATAGTAGAG-3′ (IL-22R1 reverse). The human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA was used as an internal standard for template loading of PCR by using primers 5′-TCCCATCACCA TCTTCCA-3′ (GAPDH forward) and 5′-CATCACGCC ACAGTTTCC-3′ (GAPDH reverse). PCR was performed and PCR products were analyzed by 1% agarose electrophoresis with ethidium bromide staining.
Luciferase assay
Cells were plated in 24-well plates in triplicate at a density of 5 × 104 cells per well. Next day, the cells were infected with Adflt-Luc or AdCMV-Luc at 5 TCID50 per cell in DMEM with 2% FBS for 1 h and then maintained in complete medium. Forty-eight hours after infection cells were harvested and treated with 100 μl of lysis buffer. A luciferase assay (Promega, Madison, WI) and ORION microplate luminometer (Berthold Detection systems, Oak Ridge, TN) were used for the evaluation of Luc activity of infected cells. Luciferase activity was normalized by the protein concentration of the cell lysate using DC Protein Assay (Bio-Rad, Hercules, CA).
Cell migration and invasion assays
Migration assay was performed using 12mm inserts with 12.0 μM tissue culture-treated Millcell polycarbonate membranes (Millipore, Bedford, MA). Cells were harvested, washed and added to the upper chambers at concentrations of 5×104 cells per well. Recombinant VEGF (rVEGF) was added into the medium of the lower well at 100 ng ml−1 and cells were allowed to migrate for 96 h at 37 °C. After incubation nonmigrated cells on the upper side of the tissue culture plate insert were removed with a cotton swab and the migrated cells attached to the lower side of the membrane were released using trypsin digest and counted using a hemacytometer. The in vitro metastatic activity of glioma cells was measured by using the matrigel invasion assay. Briefly, transwell inserts were coated with 100 μl of Matrigel Basement Membrane Matrix (Becton Dickinson, Bedford, MA) at 1mgml−1 to form a thin continuous film on the top of the filter. Glioma cells were added in the upper chambers at a density of 5 × 105 cells per well and medium alone or supplemented with rVEGF was added to the lower chamber. Ninety-six hours later cell culture medium was removed and cells were stained and counted as described above, and the number of cells invaded and attached in the lower chamber was measured as the invasive activity. All assays were done in triplicate.
Western blot assay
The level of IL-24 protein expression was examined by immunoblotting technique. Briefly, the conditioned media was collected and centrifuged at 14 000 × g for 5 min. Cells were washed in TBS (135mM NaCl, 2.5mM KCl, 25mM Tris-HCl, pH 7.5), and homogenized in ice-cold lysis buffer (50mM Tris-HCl, 150mM NaCl, 2mM EDTA, 1% IGEPAL CA-630 (Sigma, St Louis, MO). The homogenate was centrifuged at 14 000 × g for 5 min and the protein concentration was determined by the Biuret method using the BCA Protein Assay Kit (Pierce, Rockford, IL). Each sample was denatured for 5 min at 100 °C in loading buffer and electrophoretically separated on SDS-PAGE followed by transfer to a polyvinylidene difluoride membrane (Millipore). The membrane was blocked with 5% non-fat milk (Bio-Rad) in TBS. IL-24 in the cell homogenate and conditioned media was detected using specific anti-IL-24 mouse monoclonal antibody (GenHunter, Nashville, TN), and goat anti-mouse Ig(H+L)-HRP secondary antibody (Southern Biotechnology Associates, Birmingham, AL).
Cell proliferation assay
To determine cell growth after CRAd infection, cells were seeded in 96-well plates at 5 × 103 cells per well, incubated for 24 h, and infected with CRAd vectors at 1 TCID50. After 96 h, cell culture medium was removed and surviving cells were then fixed and stained with 2% (w/v) crystal violet in 70% ethanol for 3 h at room temperature. The plates were extensively washed, air-dried and optical density was measured at 595nm using an EL 800 Universal Microplate Reader (BIO-TEK Instruments, Winooski, VT). Relative density of adherent cells was defined as OD595 treated cells in comparison with untreated cells and the OD595 value of blank wells was subtracted.
Flow cytometry analysis of apoptosis
Annexin V binding and propidium iodide (PI) uptake were used for apoptosis evaluation. D54MG cells were collected at different time points and double stained with FITC-conjugated annexin V and PI for 15 min at room temperature. Annexin V and PI were added according to the manufacturer’s recommendations (BioVision, Palo Alto, CA). Samples were immediately analyzed by FACScan. Annexin V and PI emissions were detected in the FL-1 (530/30 nm) and FL-2 (585/40 nm) channels, respectively. For each sample, data from approximately 104 cells were recorded in list mode on logarithmic scales. Analysis was performed with Cell Quest software (Becton Dickinson, San Jose, CA) on cells characterized by forward/side scatter (FSC/SSC) parameters. Cell debris characterized by a low FSC/SSC was excluded from analysis. Annexin V-positive and PI-negative cells were considered apoptotic, whereas cells that were only PI-positive were considered necrotic. The percentage of apoptotic cells was calculated.
Subcutaneous human glioma xenograft model
Female athymic nude mice were purchased from the Frederick Cancer Research Facility (Bethesda, MD) and housed under aseptic conditions in microisolator cages and experiments were carried out according to the Institutional Animal Care and Use Committee approved protocols. To assess therapeutic effects on established solid tumors, 6 × 106 D54MG glioma cells were injected subcutaneously (s.c.) into female athymic nude mice. Treatment was started 11 days post-tumor cell injection at the time of established tumor growth (tumors were 6–8mm in diameter), noted as day 0. Animals were randomly divided into groups receiving different treatments: PBS; CRAdfltRGD-IL24; PBS plus TMZ; CRAdfltRGD-IL24 plus TMZ. Tumors were injected directly with PBS or 1 × 107 TCID50 CRAdfltRGD-IL24 on days 0, 5, 9 and 14. For oral administration (p.o.), TMZ was resuspended in PBS and mice were treated with PBS or TMZ at 16 mg kg−1 of body weight on days 2, 7, 11, 15 and 18. Tumor size was monitored twice a week with digital Vernier calipers. Tumor volumes were calculated as 0.5 × length × width2 (mm3) and plotted as a percentage change over time relative to the mean size on day 0 for each group.
Real-time quantitative PCR
Quantitative analysis of the Ad fiber gene expression was performed using real-time PCR. For in vitro studies, glioma cells were infected with 1 TCID50 per cell of Ads, the cells were collected at different times after infection, and total DNA was extracted using QIAamp DNA Mini Kit (Qiagen). For in vivo studies, D54MG tumor xenografts were generated by s.c. transplantation to the flanks of female athymic nude mice as described above. Tumor xenografts were infected with 1 × 107 TCID50 of CRAdRGDflt-IL24. At different times after infection, the animals were killed and tumor tissue was harvested and template DNA was prepared from whole tissue extracts using QIAamp DNA Mini Kit (Qiagen). For preparation of control samples, CRAdRGDflt-IL24 genomic DNA was extracted from purified viral stock by using a QIAamp DNA Mini Kit. Serial 10-fold dilutions (from 1 × 107 to 1 viral particle per reaction) of CRAdRGDflt-IL24 DNA were included in each run to establish a standard curve for a quantitative appraisal of fiber target gene copy number. For detection of the Ad fiber gene, the following primers and TaqMan probe were used: Ad5-Fiber-FWD 5′-CACAGGCCCCGCTAACC-3′, Ad5Fiber-REV 5′-GGGTCCTTGGGTGGCAAT-3′ and Ad5Fiber-PROBE 5′-6FAM-CGACTCCAAACTTAG-MGBNFQ-3′. In each reaction, 20 ng of total DNA was used as a template and PCR was performed in 25 μl of reaction mixture containing 12.5 μl of 2 × Taq Man Universal PCR master Mix (PE Applied Biosystems, Foster City, CA), 300 nM each primer and 100 nM fluorogenic probe. Amplifications were carried out in a 96-well reaction plate (PE Applied Biosystems) in a spectrofluorimetric thermal cycler (ABI PRISM 7000 Sequence Detector; PE Applied Biosystems). After the initial denaturation (2 min at 95 °C), amplification was performed with 45 cycles of 15 s at 95 °C and 60 s at 60 °C. Each sample was run in triplicate. A threshold cycle (Ct) for each triplicate was estimated by determining the point at which the fluorescence exceeded a threshold limit (10-fold the s.d. of the baseline). CRAdRGDflt-IL24 production in human tumor xenografts was determined as the Ad fiber gene copy number per 1 ng total DNA.
In situ apoptosis detection by TUNEL staining
The formalin-fixed and paraffin-embedded 5-μm thick sections of all tumor samples were studied by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining by using the DeadEnd Colorimetric TUNEL System (Promega, Madison, WI). The extent of apoptosis was evaluated by counting the positive brown-stained cells as well as the total number of cells at 10 arbitrarily selected fields in a blinded manner. The apoptotic index was calculated as the number of apoptotic cells per × 200 microscope field (at least five random fields/tumor).
Immunohistochemical analysis of tumors for CD31 expression
Sections from paraffin-embedded tumors (those used for TUNEL staining) were incubated overnight with rat anti-mouse CD31 polyclonal antibody. Then sections were incubated with donkey anti-rat antibody secondary antibody. Antigen–antibody complexes were visualized by incubation with 3,3′-diaminobenzidine substrate and counterstained with diluted Harris hematoxylin. Tumor vessels containing CD31-positive (brown) cells were quantified by microscopy (original magnification, × 200) of at least five random fields/tumor, and were calculated as relative vessel density.
Intracranial survival study
To establish orthotopic D54MG tumor xenografts, athymic nude mice were anesthetized by intraperitoneal cavity (i.p.) administration of ketamine HCl and xylazine mixture in saline at 140 and 2.1 mg kg−1 of body weight, respectively. A midline scalp incision was made, and a 0.5-mm burr hole was drilled 1.5–2.0mm to the right of midline and 1.0mm anterior to the coronal suture. D54MG cells were injected stereotactically at 5 × 104 cells, using a 250 μl Hamilton syringe with a prepared 30-G needle mounted in a Stoelting stereotaxic apparatus. A plastic sleeve surrounding the needle allowed reproducible injections of tumor cells, saline or CRAdRGDflt-IL24 to a depth of 2.5 mm. The needles were left in place for 2 min to minimize reflux of the tumor cells along the needle track. The scalp wounds were closed with Tissu-Mend glue to avoid scatter dose from metallic wound clips during radiation treatment. The mice were placed on a heating pad in sterile microisolator polycarbonate cages and allowed to awaken from anesthesia. Tumors were allowed to grow for 5 days before the start of treatment (day 0). Animals were divided into groups receiving different treatments: PBS; CRAdRGDflt-IL24 plus PBS; PBS plus TMZ; CRAdRGDflt-IL24 plus TMZ. Two days after an intratumoral injection of sterile PBS or 1 × 107 TCID50 of CRAdfltRGD-IL24 in 10 μl PBS, mice were treated p.o. with either PBS or TMZ at 12.5mg kg−1 and monitored daily for survival. When tumor-bearing mice displayed overt signs of neurological dysfunction, manifested primarily as a hunched appearance, lack of grooming and lack of avoidance behavior when handled, they were killed by lethal CO2 inhalation and their brains were harvested for histopathological examination, confirming the presence of progressive tumor in all dead mice.
Statistical analysis
All error terms are expressed as the s.d. of the mean. Significance levels for comparison of differences between groups in the in vitro experiments were analyzed by a Student’s t-test. The differences were considered significant when P-value was <0.05. All reported P-values are two-sided. In the animal model tumor therapy studies, the treatment groups were compared with respect to tumor size and percent of original tumor size over time. To test for significant differences in mean time to tumor doubling between treatment groups, one-way analysis of variance (ANOVA) test was conducted. When the ANOVA indicated that a significant difference existed (P-value <0.05), multiple comparison procedures were used to determine where the differences lay. Kaplan–Meier survival curves were analyzed by the log-rank test, and specific pairwise multiple comparisons were made using the Holm–Sidak method. All comparisons were made using the 0.05 level of significance.
Results
VEGFR-1 promoter activity in glioma cell lines
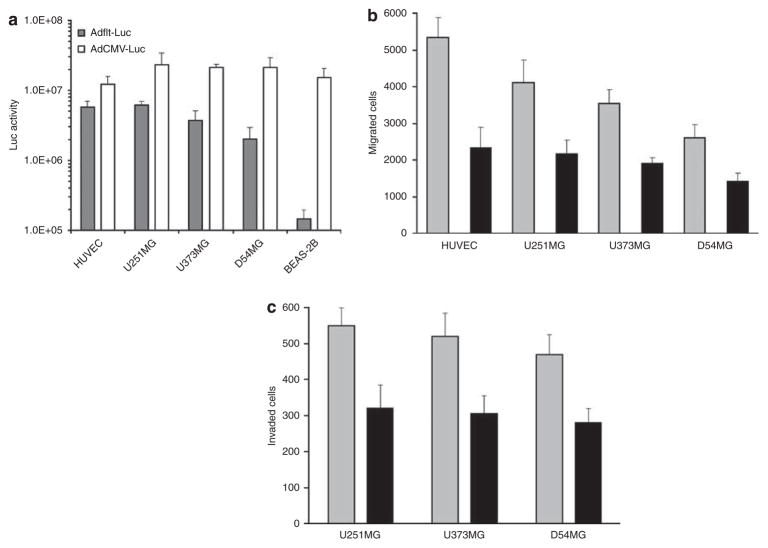
Cancer cell specificity remains a serious obstacle in CRAd-mediated gene therapy. Transcriptional targeting could add a further level of safety to regulate CRAd propagation selectively in tumor cells by using specific promoters for the expression of essential adenoviral genes, including E1 early region. To determine the capacity of the VEGFR-1/flt-1-targeted CRAd vector to selectively replicate in glioma cells, we evaluated the ability of the VEGFR-1 promoter to drive the cell-specific gene expression. Human glioma cells were infected with replication-deficient recombinant adenoviruses, Adflt-Luc or AdCMV-Luc, encoding the firefly luciferase (Luc) gene under control of the VEGFR-1 or CMV promoter, respectively. Forty-eight hours after infection, cells were harvested and firefly luciferase expression was analyzed using a luciferase assay system (Figure 1a). Differing levels of Luc expression were detected in the tested cell lines, the level of expression increasing in proportion to the multiplicity of infection (results not shown). As expected, high levels of relative expression of VEGFR-1-driven Luc were observed in HUVEC endothelial cells in comparison with BEAS-2B normal bronchial epithelial cells (48.4±6.8 and 0.9±1.1%, respectively, compared with AdCMV-Luc). Although all four glioma cell lines expressed VEGFR-1 mRNA (data not shown), these cells demonstrated wide variation in VEGFR-1 promoter activity. The levels of VEGFR-1 driven Luc expression were relatively high in U251MG (26.9±5.9%), intermediate in U373MG (18.7±3.9%) and low in D54MG cells (8.6±2.2%) compared with AdCMV-Luc.
Figure 1.
VEGF increases migration and metastatic activity of glioma cells in vitro. (a) VEGFR-1 promoter activity in glioma cell lines. Glioma cells, BEAS-2B normal bronchial epithelial cells (negative control) and HUVEC (positive control) were infected with Adflt-Luc or AdCMV-Luc (control of infectivity) at 5 TCID50 per cell. Luciferase expression was analyzed at 48 h after infection by luciferase assay system. Luc activity expressed in relative luciferase units after subtraction of uninfected control cells. Presented are mean values±s.d. of three independent experiments. (b) VEGF-stimulated migration of glioma cells. Glioma cells and human endothelial cells were seeded in the upper chamber of a tissue culture plate insert. Then, 100 ng ml−1 of rVEGF was added into the medium of cells in the lower compartment and cells were allowed to migrate for 96 h. The migrated cells attached to the lower chamber were counted using a hemacytometer. Data presented are mean values±s.d. of the number of migrated cells in the presence (gray) or without rVEGF (black) in three independent experiments, each performed in triplicate. (c) VEGF increased glioma cell invasion in vitro. Glioma cells were seeded in the upper chamber coated with Matrigel. Later, 100 ng ml−1 of rVEGF was added into the medium of cells in the lower chamber and they were allowed to migrate for 96 h. The migrated cells attached to the lower chamber were counted using a hemacytometer. Data presented are mean values±s.d. of the number of migrated cells in the presence (gray) or without rVEGF (black) in three independent experiments, each performed in triplicate.
VEGF increases glioma cell metastatic activity and proliferation rate
Based on the data that demonstrated moderate levels of VEGFR-1 promoter activity and mRNA expression in glioma cells, we evaluated the regulatory effect of VEGF on glioma cell migration and replication. The in vitro motility of glioma cells was assessed using migration across a porous membrane in the presence of recombinant VEGF165 (rVEGF). As shown in Figure 1b, rVEGF at 100 ng ml−1 stimulated migration of HUVEC, U251MG, U373MG and D54MG cells by 2.3-, 1.9-, 1.8- and 1.8-fold, respectively (P-values <0.05). Importantly, levels of cell migration correlated with VEGFR-1 promoter activity, U251MG cells with high VEGFR-1 promoter activity demonstrated significantly enhanced basal as well as rVEGF-stimulated migration compared with D54MG cells (1.5-fold; P=0.04 and 1.6-fold; P=0.03, respectively). Similarly, rVEGF also increased invasion of U251MG, U373MG and D54MG cells across the porous membrane coated with reconstituted basement membrane, by 1.8-, 1.7- and 1.6-fold, respectively (P-values <0.05; Figure 1c).
To determine the effect of VEGF on cell growth, U251MG and D54MG cells were seeded in 6-well plates at 1 × 105 cells per well, and incubated in growth media with rVEGF at 100 ng ml−1. Seventy-two hours later glioma cells were collected using trypsin digest and counted using a hemacytometer. The cell proliferation rate was calculated as time for doubling the number of cells. The addition of rVEGF in cell culture growth media decreased the mean time to tumor cell doubling in comparison with growth media without rVEGF as follows: U251MG cells from 21.4±1.4 to 18.6±0.8 h, respectively (P=0.04) and for D54MG cells from 23.6±1.2 to 20.4±0.9 h, respectively (P=0.02).
CRAdRGDflt-IL24 produces increased human glioma cell killing in vitro
The main factor currently limiting the clinical potential of gene therapy of glioma is the poor level of in situ tumor cell transduction achievable by existing gene transfer vectors. Thus, oncolytic CRAd vectors are particularly attractive due to their susceptibility to infect and efficiently proliferate in a wide variety of cell types within the central nervous system. However, a major drawback of CRAd-mediated gene therapy is that most tumor cells demonstrate low levels of expression of the primary CAR receptor for adenovirus (Ad).
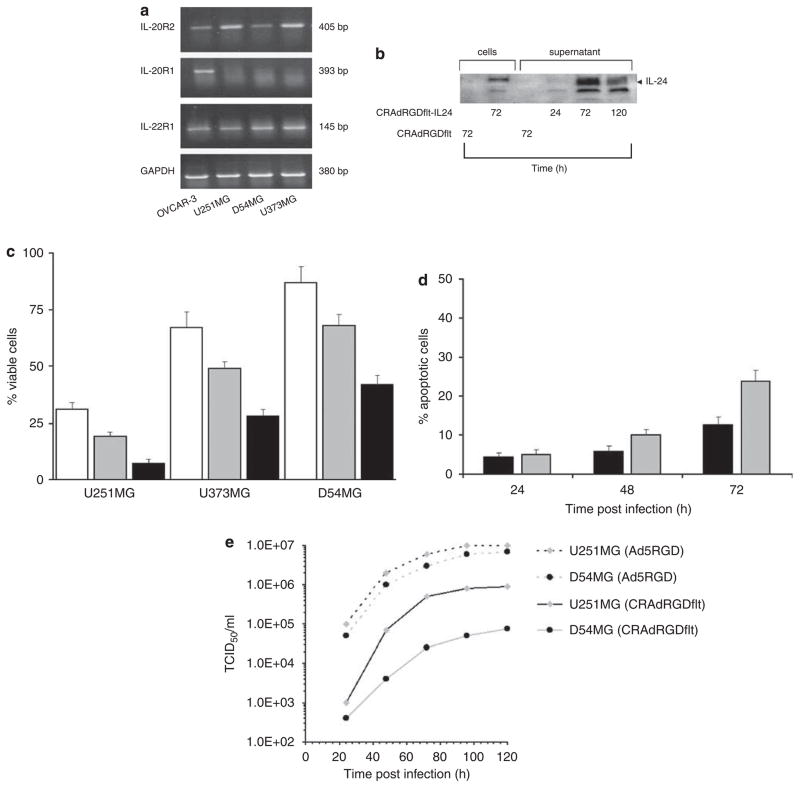
To address these limitations, we have developed retargeted CRAd employing the VEGFR-1 promoter to control E1a gene expression with a CDCGRDCFC peptide inserted into the HI-loop of the Ad fiber knob domain and encoding the IL-24 gene (CRAdRGDflt-IL24). Initially, IL-24 receptor expression in human glioma cells was investigated by RT-PCR. As shown in Figure 2a, all tested glioma cell lines expressed IL-22R1 and IL-20R2 mRNA (type 2 IL-20R). D54MG cells showed low level IL-20R2 mRNA expression, and undetectable levels of IL-20R1 in comparison with OVCAR-3 cells (positive control) as measured by RT-PCR.
Figure 2.
CRAdRGDflt-IL24 produces increased cytotoxicity in vitro. (a) IL-24 receptor mRNA expression in human glioma cell lines. Human glioma cells and OVCAR-3 human ovarian cancer cells (positive control) were collected and total RNA was extracted. The levels of IL-20R2, IL20R1 and IL-22R1 and GAPDH (loading control) mRNA expression were determined using RT-PCR. (b) CRAdRGDflt-IL24 produces high levels of IL-24 expression in glioma cells. The expression of IL-24 protein in cell lysates and conditioned media of D54MG cells infected with 1 TCID50 per cell of CRAdRGDflt-IL24 or CRAdRGDflt (negative control) at 24, 72 and 120 h after infection was examined using western blot analysis. (c) Combination of RGD modification of Ad fiber and IL-24 expression increases CRAd-mediated oncolysis of glioma cells. Glioma cells were infected with 1 TCID50 per cell of CRAdflt (white), CRAdfltRGD (gray) or CRAdRGDflt-IL24 (black). Cell viability was determined at 96 h after infection by using the crystal violet inclusion assay. Data shown in comparison with uninfected control cells. Presented are mean values±s.d. of three independent experiments. (d) CRAdRGDflt-IL24 enhances glioma cell apoptosis. After infection with 1 TCID50 per cell CRAdRGDflt (black) or CRAdRGDflt-IL24 (gray), D54MG cells were examined for annexin V binding and PI uptake. Cells were collected and double stained with FITC-conjugated annexin V and PI. Cells taking up vital dye PI were considered necrotic, annexin V-positive cells were considered apoptotic and their percentages were calculated. Samples were analyzed by FACScan. Data expressed as percentage of annexin V-positive cells are the means after subtraction of uninfected control cells. Presented are mean values±s.d. of three independent experiments. (e) CRAdRGDflt-IL24 proliferation in glioma cells in vitro. Glioma cells were infected with 1 TCID50 per cell of CRAdRGDflt-IL24 or AdRGD (infectivity control) and viral titers were measured by a TCID50 assay at different times after infection.
To determine the levels of IL-24 protein expression in vitro, D54MG cells were infected with 1 TCID50 per cell of CRAdRGDflt-IL24 or CRAdRGDflt. The conditioned media and cells were collected at 24, 72 and 120 h after infection and subjected to western blot analysis. The results showed that IL-24 protein was present in D54MG cells and conditioned media after CRAdRGDflt-IL24 infection (Figure 2b). IL-24 expression was not detectable in conditioned media or in cell lysates of CRAdRGDflt-infected cells (negative control) using immunoblotting technique.
To assess the biological activity of CRAd-mediated oncolytic therapy, glioma cells were infected with 1 TCID50 per cell of CRAdRGDflt-IL24, CRAdRGDflt or CRAdflt and cell viability was determined 96 h after infection using crystal violet staining. As shown in Figure 2c, the relative sensitivity of glioma cells to CRAdflt infection correlated with VEGFR-1 promoter activity. The insertion of a CDCGRDCFC peptide into the HI-loop of the Ad fiber knob domain increased CRAdRGDflt-mediated glioma cell killing. Also, these results demonstrated that IL-24 overexpression significantly increased CRAdRGDflt-IL24-mediated cytotoxicity in comparison with CRAdRGDflt.
To evaluate mechanisms of CRAdRGDflt-IL24-mediated cytotoxicity, D54MG cells were infected with CRAdRGDflt-IL24 or CRAdRGDflt and then collected at 24, 48 and 72 h after infection. Cells were double stained with PI and FITC-labeled annexin V and their emission was analyzed by flow cytometry (Figure 2d). An increase in annexin V-positive cells was observed at 48–72 h compared with untreated cells after infection with CRAdRGDflt-IL24 and CRAdRGDflt (P-values <0.05). There was significantly greater annexin V-positive staining for D54MG cells infected with CRAdRGDflt-IL24 versus those infected with CRAdRGDflt (P=0.02) at 72 h, but not at 24 or 48 h after infection (P-values ≥0.05). The number of annexin V- and PI-positive cells increased 72 h after infection with CRAdRGDflt-IL24 (data not shown).
To confirm whether CRAdRGDflt-IL24 propagation in glioma cells correlated with VEGFR-1 promoter activity, U251MG and D54MG cells with high and low VEGFR-1 promoter activity, respectively, were infected with 1 TCID50 per cell of CRAdRGDflt-IL24 or Ad5RGD (wild type Ad5 with a CDCGRDCFC modified the Ad fiber), cells were collected at different times after infection and viral titer was measured by a TCID50 assay. As seen in Figure 2e there was a time-dependent increase of CRAdRGDflt-IL24 titer in both glioma cell lines. There were significantly greater viral titers for U251MG cells in comparison with D54MG cells infected with CRAdRGDflt-IL24 (P-values <0.05) at 48, 72, 96 and 120 h after infection, but no significant differences between those cells infected with Ad5RGD (P-values >0.05).
Combination of CRAdfltRGD-IL24-mediated therapy with chemotherapy in vitro
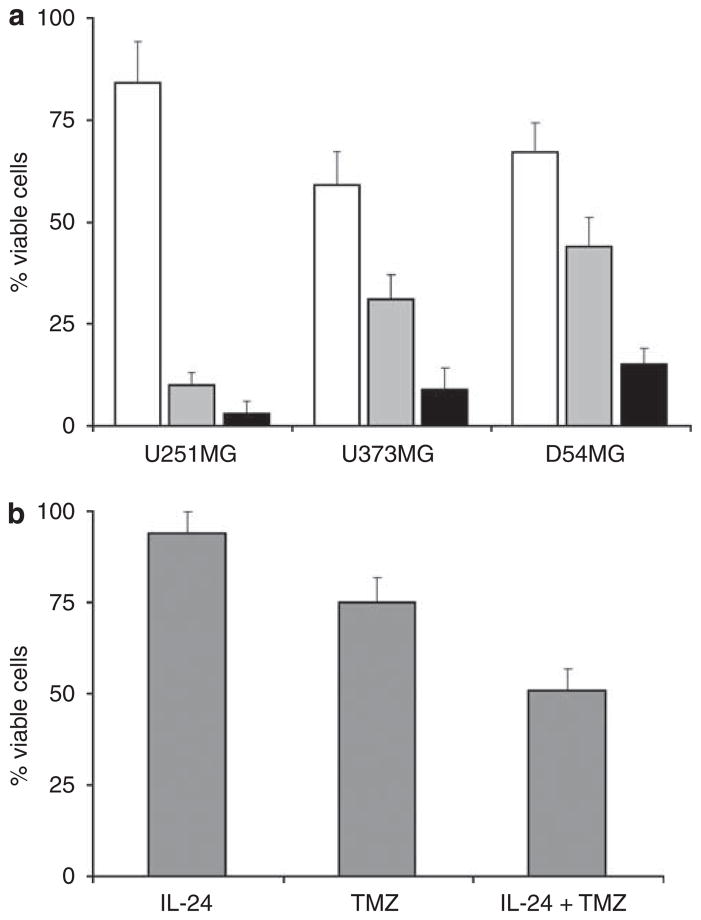
A series of studies were carried out to clarify whether chemotherapy can increase CRAdRGDflt-IL24-mediated oncolytic therapy in a panel of glioma cells. Cells were infected with 1 TCID50 per cell of CRAdRGDflt-IL24 and TMZ was added in culture media 24 h after infection in a final concentration at 50 μM. The results of the cytotoxicity assays summarized in Figure 3a show that the combination of CRAdRGDflt-IL24 and TMZ produced significantly enhanced glioma cell killing in comparison with either treatment alone. To investigate the effect of IL-24-mediated cytotoxicity in combination of TMZ, D54MG cells were incubated with TMZ and human recombinant IL-24 protein and cell viability was evaluated by crystal violet staining. As shown in Figure 3b, the combination of TMZ and IL-24 protein produced significantly increased cell killing in comparison with these treatments alone. Increased cytotoxicity following combined treatment with IL-24 and TMZ was associated with an enlarged number of annexin V-positive cells in comparison with the number after treatment with these agents alone, measured by flow cytometry analysis using double staining with PI and FITC-labeled annexin V (data not shown).
Figure 3.
Combination of CRAdRGDfltIL-24 and TMZ increases glioma cell killing. (a) Glioma cells were incubated with 50 μM of TMZ (white), infected with 1 TCID50 per cell of CRAdRGDfltIL-24 (gray), or received the combination of CRAdRGDfltIL-24 with TMZ (black). Cell viability was determined at 96 h after treatment by using the crystal violet inclusion assay. The percentage of viable cells is shown in comparison with uninfected control cells. Data presented are the mean values±s.d. of three independent experiments. (b) Combination of IL-24 protein with TMZ increases glioma cell killing. D54MG cells were incubated with 10mg of recombinant human IL-24 protein, 50 μM of TMZ or received the combination of IL-24 protein and TMZ. Cell viability was determined at 96 h after treatment by using the crystal violet inclusion assay. The percentage of viable cells is shown in comparison with uninfected control cells. Data presented are the mean values±s.d. of three independent experiments.
Subcutaneous glioma therapy study
Taking into consideration the results of previous experiments, D54MG glioma cells were selected for subsequent animal studies because they demonstrate the lowest levels of VEGFR-1 promoter activity and intermediate resistance for in vitro treatment with TMZ, and thus provided the most stringent test of the efficacy of CRAdfltRGD-IL24- mediated therapy.
To appraise levels of CRAdRGDflt-IL24 replication, D54MG s.c. human tumor xenografts were injected with PBS or 1 × 107 TCID50 CRAdRGDflt-IL24 (six animals per group). Tumors were collected at 6, 72 and 168 h after single injection, total DNA was extracted and CRAdRGDflt-IL24 propagation was measured using real-time PCR. The mean number of Ad fiber gene copies was increased 35- and 4-fold at 72 and 168 h after treatment in comparison with data obtained at 6 h following CRAdRGDflt-IL24 injection, respectively. There were significant differences (P-values<0.05) across time for Ad fiber gene copies following injection of D54MG tumor xenografts with CRAdRGDflt-IL24 (data not shown).
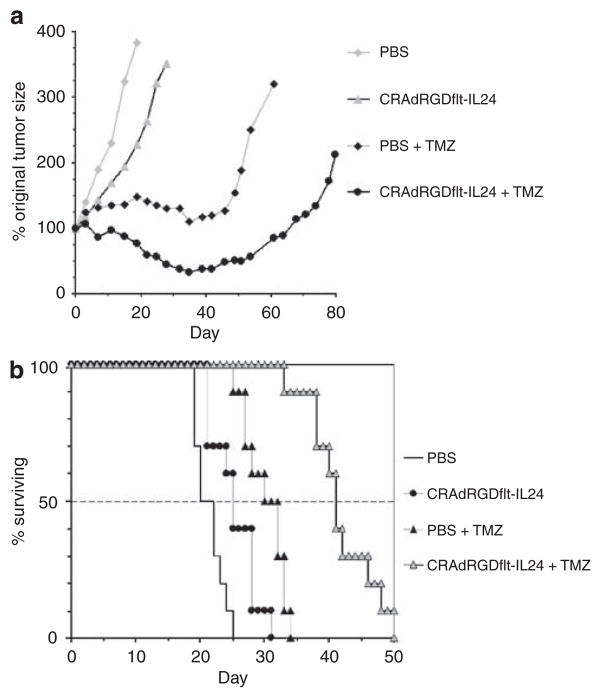
To further evaluate the therapeutic potential of combination oncolytic virotherapy with chemotherapy in vivo, D54MG cells were subcutaneously (s.c.) injected into the flank of athymic nude mice. Before treatment, the mean tumor sizes in groups of 10 mice at baseline were not significantly different between treatment groups (P>0.05), and the within treatment variances were not significantly different (P>0.05). The baseline mean and s.d. for tumor volumes at 11 days post-tumor cell injection was 189.4±75.7mm3. In vivo tumor therapy was initiated on day 0, which corresponded to 11 days post-tumor cell injection. Animals were injected i.t. with PBS or 1 × 107 TCID50 CRAdfltRGD-IL24 on days 0, 5, 9 and 14. Temozolomide was administered p.o. at 16mg kg−1 of body weight on days 2, 7, 11, 15 and 18 (Figure 4a). The mean time to tumor volume doubling for PBS alone, CRAdfltRGD-IL24 alone, PBS plus TMZ, CRAdfltRGD-IL24 plus TMZ groups were 8, 17, 52 and 79 days, respectively. Comparisons of mean time to tumor volume doubling of the group treated with CRAdfltRGD-IL24 alone versus PBS alone, CRAdfltRGD-IL24 alone versus PBS plus TMZ and CRAdfltRGD-IL24 plus TMZ versus PBS plus TMZ showed significant differences between the groups (P-values <0.05).
Figure 4.
Efficacy of combination CRAdRGDflt-IL24 oncolytic virotherapy with chemotherapy in human glioma xenograft models. (a) Subcutaneous glioma therapy study. Treatment was started at the time of established tumor growth (day 0 equal to 11 days after tumor cell injection). Animals were injected i.t. with PBS or 1 × 107 TCID50 CRAdRGDfltIL-24 on days 0, 5, 9 and 14. Temozolomide (TMZ) was administered p.o. at 16mgkg−1 of body weight on days 2, 7, 11, 15 and 18. Data points represent the mean change in tumor volume relative to day 0 for each group of animals. (b) Intracranial glioma therapy study. D54MG human glioma cells (5 × 104 cells per mouse) were injected into the right frontal cortex of athymic nude mice. Five days after tumor implantation, a single dose of PBS or 1 × 107 TCID50 of CRAdfltRGD-IL24 was injected i.t. Mice then received p.o. PBS or TMZ at 12.5mgkg−1 on day 7 and were subsequently monitored daily for survival.
To assess the in vivo effect of CRAdRGDflt-IL24 on apoptosis, the tumor samples were analyzed by terminal deoxynucleotidyl TUNEL. D54MG s.c. xenograft-bearing mice received treatment with CRAdRGDflt-IL24, as described above, and 3 days after the last injection, the tumors were excised, fixed in 10% neutral-buffered formalin and stained for apoptosis. Microscopic examination of TUNEL-stained tumor sections showed that compared with PBS-injected control mice, CRAdRGDflt-IL24 injection increased the number of positive cells. The quantitative evaluation of apoptosis showed that the number of TUNEL-positive cells in D54MG tumor xenografts treated with CRAdRGDflt-IL24 increased 4.6-fold (P<0.05) over that of PBS-treated control tumors (Table 1).
Table 1.
The effects of oncolytic CRAdRGDfltIL-24-mediated virotherapy on apoptosis and tumor blood microvessel density of tumor xenografts
| Treatment | TUNEL-positive cells (%) | CD31-positive vessel density |
|---|---|---|
| PBS | 3.8±1.7 | 32±4.1 |
| CRAdRGDfltIL-24 | 17.5±3.6a | 11±2.4a |
P<0.05 in comparison with PBS-treated control.
To determine whether the inhibitory effect of CRAdflt-IL24 on tumor growth was associated with the suppression of tumor angiogenesis, the distribution of the endothelial cell-specific antigen CD31 was examined. Immunohistochemical analysis of endothelial cells in D54MG tumors treated with anti-CD31 antibody showed fewer stained CD31-positive endothelial cells and 2.9-fold reduced microvessel density (P<0.05) in a cross-section of tumors from mice treated with CRAdRGDflt-IL24 (Table 1) compared with PBS-treated tumor control. These results demonstrate the pro-apoptotic and anti-angiogenesis activity of CRAdRGDflt-IL24 virotherapy of glioma tumor xenografts.
Intracranial glioma therapy study
The potential clinical efficacy of oncolytic virotherapy using CRAdfltRGD-IL24 was assessed in an orthotropic mouse model. Five days after intracranial implantation of D54MG cells, animals received i.t. injection of PBS or 1 × 107 TCID50 of CRAdfltRGD-IL24 and 2 days afterward PBS or TMZ at 12.5 mg kg−1 were administered p.o. As shown in Figure 4b, the median survival times for PBS alone, CRAdfltRGD-IL24 alone, PBS plus TMZ and CRAdfltRGD-IL24 plus TMZ were 20, 25, 30 and 41 days, respectively. CRAdfltRGD-IL24 in combination with TMZ significantly prolonged survival of mice in comparison to PBS alone, CRAdfltRGD-IL24 alone or TMZ alone (P-values <0.05). Also, TMZ plus PBS significantly prolonged survival of mice in comparison with PBS alone (P<0.05).
Discussion
The concept of utilizing an intratumoral or intracavitary injection of oncolytic vectors as a therapeutic tool is particularly attractive in glioma treatment. Although preclinical studies using various genetically engineered Ad or herpes simplex virus 1 vectors have demonstrated promising results, the difficulty in replicating the experimental work in therapy studies of patients with glioma has been disappointing. Oncolytic CRAd vectors are attractive due to their susceptibility to infect and efficiently proliferate in a wide variety of cell types within the central nervous system. There are several approaches to develop effective CRAd-mediated virotherapy including use of specific promoters for targeting of CRAd replication, genetic modifications of Ad capsid for CARindependent infection and incorporation of therapeutic genes that can enhance oncolytic activity of CRAd vectors.
It was shown that Ad transduction efficiency to both established and primary human glioma cells in vitro is highly variable, and gene transfer levels correlated directly with the level of cell surface CAR.23 Thus, CAR expression may be a major limiting factor to the success of Ad-based cancer gene therapy, particularly for malignant gliomas. As only a subset of patients may have tumors that are efficiently infected by Ad vectors upon intralesional injection, the utilization of retargeted CRAd vectors by expression of RGD in the fiber knob may prove extremely useful in producing maximum benefit from CRAd-based gene therapy.15,16,24
Tumor blood vessel endothelial cells are in direct contact with the circulation. Each endothelial cell supplies oxygen and nutrients to many thousands of tumor cells. Thus, vascular-targeted anticancer therapy is an attractive strategy for glioma. The newly formed tumor vasculature differs remarkably from mature vessels in normal tissues. High levels of VEGF production in glioma cells and robust expression of its cognate receptors in tumor-associated blood microvessels suggest that VEGF/VEGF receptor expression plays an important role in glioma-induced angiogenesis. A variety of studies have shown the importance of VEGF and VEGFR expression as a prognostic indicator of the severity of brain cancer.25–28 Rapid growth of gliomas results in focal ischemia and hypoxia, which in turn induces microvascular hyperplasia by VEGF activity. This assumption is based predominantly on observations of increased VEGF expression in perinecrotic regions and at the invasion zone between tumor and normal brain tissue.3 The overexpression of VEGF in cancer cells promotes tumor angiogenesis by activation of VEGF receptor expression in tumor vessels compared with surrounding normal endothelial cells.5–8
Consistent with reports by others,17–19 our experimental data provide evidence of relatively high levels of VEGFR-1 promoter activity in blood vessel endothelial cells and in certain established glioma cell lines. The results of in vitro studies demonstrate direct correlation of VEGFR-1 promoter activity with levels of VEGF-stimulated migration and proliferation of glioma cells. These findings suggest that co-expression of VEGF and its receptors by brain tumor cells raises the possibility of autocrine stimulation of malignant glioma development and dissemination.9–12 The CRAdRGDflt-IL24-mediated virotherapy was associated with suppression of tumor blood microvascular hyperplasia and activation of apoptosis. These results demonstrate that CRAdRGDflt-IL24 infection causes selective killing of blood vascular endothelial cells and VEGFR-1-positive tumor cells.
In this study, CRAdRGDflt-IL24 has been engineered to function as a therapeutic gene delivery vehicle by incorporating an expression cassette containing the IL-24 transgene to improve the antitumor efficacy of oncolytic virotherapy. A growing body of evidence demonstrates that Ad-mediated expression of IL-24 results in growth inhibition in malignant gliomas irrespective of p53 status, in contrast to normal human non-transformed primary astrocytes.21,22 IL-24 gene expression selectively induces G2/M arrest and apoptosis in cancer cells by promoting mitochondrial dysfunction and reactive oxygen species production, as well as an alteration of the ratio of pro- to anti-apoptotic Bcl-2 protein family members.20,29 In addition to its direct effect on cancer cells resulting in apoptosis, the IL-24 protein acts as a cytokine and has shown antiangiogenic activity. IL-24 inhibited both endothelial cell differentiation and migration of endothelial cells induced by VEGF and basic fibroblast growth factor.30,31 IL-24 protein apparently signals through two heterodimeric receptors, the IL-22R1/IL-20R2 and the IL-20R1/IL-20R2. Results of RT-PCR analysis of expression of IL-24 receptor demonstrate that glioma cells express IL-20R type 2 (comprising IL-22R1 and IL-20R2) receptor. The same type of IL-24 receptor mRNA was detected by RT-PCR using an extended panel of glioma cell lines (data not shown). Importantly, previous studies demonstrated that antiangiogenic activity of IL-24 expression was mediated through IL-22 receptor in HUVECs.30 Also, IL-24 gene expression inhibited migration and invasion of human lung cancer by down-regulating the production of phosphatidylinositol 3-kinase/protein kinase B, focal adhesion kinase and matrix metalloproteinase-2 and -9.32
The results also demonstrated that the relative sensitivity of glioma cells to CRAd-mediated virotherapy is associated with levels of CRAdRGDflt-IL24 proliferation in glioma cells and tumor xenografts, which directly correlated with VEGFR-1 promoter activity. To investigate the mechanisms involved in CRAdRGDflt-IL24-mediated cell killing, the combined staining with fluorescein-conjugated annexin V and PI was used. The exposure of phosphatidylserine on the outside of the plasma membrane is an early event characteristic of the apoptotic process, prior to the loss of integrity of the plasma membrane (necrosis). The results indicate that CRAdRGDflt-IL24 rapidly induced apoptosis and then necrosis at a later time.
The goal of cancer treatment has been to trigger tumor-selective cell death. Although cell death can be achieved not only by apoptosis but also by necrosis, mitotic catastrophe, as well as autophagy, the main chemotherapeutic agents act by regulation of the apoptosis-related signaling pathways. However, apoptosis inhibition resulted in the inherent resistance of malignant glioma to chemotherapy.33
It is becoming more evident from clinical studies on conventional chemotherapy that multimodality treatment strategies have a higher chance of success, presumably because combined therapies simultaneously utilize diverse target-signaling pathways in tumor cells. Owing to the high intrinsic resistance of malignant glioma cells against DNA-damaging drugs and the failure of many chemotherapy agents to cross the blood–brain barrier, only a few drugs proved to be active against malignant glioma in clinical settings including TMZ, the DNA methylating agent with a predisposition to guanine.33–37 It was shown that TMZ-mediated glioma cytotoxicity is a result of apoptosis induction and caspase-9, -2, -7 and -3 activation as well as a decrease in pro-survival Bcl-2.38 Also, glioma cells respond to TMZ therapy by undergoing G2/M arrest, and ultimately die from autophagy.39 The results of in vitro and animal therapy studies showed that CRAdRGDflt-IL24 increased the antitumor effect of chemotherapy using TMZ. Thus, apoptosis as well as autophagy share common pathways that either link or polarize the cellular responses to combined therapy using CRAdRGDflt-IL24 and TMZ. These findings are in agreement with other data showing a synergistic therapeutic effect between oncolytic Ad and TMZ.40 Cancer therapy depends on the balance between efficacy and toxicity. The combination of CRAd virotherapy with chemotherapy should provide a major advantage to achieve a higher therapeutic result by using low doses of oncolytic virus and chemotherapy.
In conclusion, we used an infectivity-enhanced flt-1 promoter-based CRAd vector with the ability to produce IL-24 to selectively induce cell death in both tumor blood vessels and glioma cells. The application of such strategies of combination chemotherapy and oncolytic virotherapy with dual action employing apoptosis-inducing and angiogenesis-suppressing IL-24 expression, offers unique opportunities to develop a more effective glioma treatment regimen.
Acknowledgments
We thank Sally B Lagan for assistance in preparing the paper. Supported in part by NCI SPORE in Brain Cancer Grant P50 CA97247.
References
- 1.Barzon L, Boscaro M, Palu G. Endocrine aspects of cancer gene therapy. Endocr Rev. 2004;25:1–44. doi: 10.1210/er.2002-0035. [DOI] [PubMed] [Google Scholar]
- 2.Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 3.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 4.Lopes MB. Angiogenesis in brain tumors. Microsc Res Tech. 2003;60:225–230. doi: 10.1002/jemt.10260. [DOI] [PubMed] [Google Scholar]
- 5.Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 6.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 7.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 8.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 9.Omura T, Miyazawa K, Ostman A, Heldin CH. Identification of a 190-kDa vascular endothelial growth factor 165 cell surface binding protein on a human glioma cell line. J Biol Chem. 1997;272:23317–23322. doi: 10.1074/jbc.272.37.23317. [DOI] [PubMed] [Google Scholar]
- 10.Chan AS, Leung SY, Wong MP, Yuen ST, Cheung N, Fan YW, et al. Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol. 1998;22:816–826. doi: 10.1097/00000478-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gesundheit B, Klement G, Senger C, Kerbel R, Kieran M, Baruchel S, et al. Differences in vasculature between pilocytic and anaplastic astrocytomas of childhood. Med Pediatr Oncol. 2003;41:516–526. doi: 10.1002/mpo.10308. [DOI] [PubMed] [Google Scholar]
- 12.Steiner HH, Karcher S, Mueller MM, Nalbantis E, Kunze S, Herold-Mende C. Autocrine pathways of the vascular endothelial growth factor (VEGF) in glioblastoma multiforme: clinical relevance of radiation-induced increase of VEGF levels. J Neurooncol. 2004;66:129–138. doi: 10.1023/b:neon.0000013495.08168.8f. [DOI] [PubMed] [Google Scholar]
- 13.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 14.Fulda S, Debatin KM. Targeting apoptosis pathways in cancer therapy. Curr Cancer Drug Targets. 2004;4:569–576. doi: 10.2174/1568009043332763. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi N, Mizuguchi H, Hosono T, Ishii-Watabe A, Uchida E, Utoguchi N, et al. Efficient gene transfer by fiber-mutant adenoviral vectors containing RGD peptide. Biochim Biophys Acta. 2001;1568:13–20. doi: 10.1016/s0304-4165(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 16.Zheng S, Ulasov IV, Han Y, Tyler MA, Zhu ZB, Lesniak MS. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–160. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- 17.Nicklin SA, Reynolds PN, Brosnan MJ, White SJ, Curiel DT, Dominiczak AF, et al. Analysis of cell-specific promoters for viral gene therapy targeted at the vascular endothelium. Hypertension. 2001;38:65–70. doi: 10.1161/01.hyp.38.1.65. [DOI] [PubMed] [Google Scholar]
- 18.Nicklin SA, Dishart KL, Buening H, Reynolds PN, Hallek M, Nemerow GR, et al. Transductional and transcriptional targeting of cancer cells using genetically engineered viral vectors. Cancer Lett. 2003;201:165–173. doi: 10.1016/j.canlet.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Work LM, Ritchie N, Nicklin SA, Reynolds PN, Baker AH. Dual targeting of gene delivery by genetic modification of adenovirus serotype 5 fibers and cell-selective transcriptional control. Gene Therapy. 2004;11:1296–1300. doi: 10.1038/sj.gt.3302292. [DOI] [PubMed] [Google Scholar]
- 20.Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, et al. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–S37. [PubMed] [Google Scholar]
- 21.Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, et al. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14:35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 22.Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–1180. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 23.Miller CR, Buchsbaum DJ, Reynolds PN, Douglas JT, Gillespie GY, Mayo MS, et al. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998;58:5738–5748. [PubMed] [Google Scholar]
- 24.Schnell O, Krebs B, Wagner E, Romagna A, Beer AJ, Grau SJ, et al. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008;18:378–386. doi: 10.1111/j.1750-3639.2008.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 26.Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59:520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 27.Carroll RS, Zhang J, Bello L, Melnick MB, Maruyama T, Mc LBP. KDR activation in astrocytic neoplasms. Cancer. 1999;86:1335–1341. doi: 10.1002/(sici)1097-0142(19991001)86:7<1335::aid-cncr32>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Yao Y, Kubota T, Sato K, Kitai R, Takeuchi H, Arishima H. Prognostic value of vascular endothelial growth factor and its receptors Flt-1 and Flk-1 in astrocytic tumours. Acta Neurochir (Wien) 2001;143:159–166. doi: 10.1007/s007010170122. [DOI] [PubMed] [Google Scholar]
- 29.Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, et al. MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Mol Cancer Ther. 2003;2:623–632. [PubMed] [Google Scholar]
- 30.Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- 31.Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther. 2004;9:818–828. doi: 10.1016/j.ymthe.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther. 2004;9:510–518. doi: 10.1016/j.ymthe.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14:2900–2908. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 35.Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 36.Jendrossek V, Belka C, Bamberg M. Novel chemotherapeutic agents for the treatment of glioblastoma multiforme. Expert Opin Investig Drugs. 2003;12:1899–1924. doi: 10.1517/13543784.12.12.1899. [DOI] [PubMed] [Google Scholar]
- 37.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 38.Kanzawa T, Bedwell J, Kondo Y, Kondo S, Germano IM. Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg. 2003;99:1047–1052. doi: 10.3171/jns.2003.99.6.1047. [DOI] [PubMed] [Google Scholar]
- 39.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 40.Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67:11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]