Table 1. Optimization of the Cu-transannulation reaction conditions a .
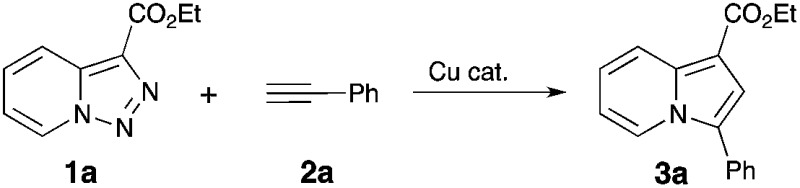
| |||
| Entry | Catalyst, mol% | T (°C) | Yield b |
| 1 | CuCl, 15% | 100 | N.R. |
| 2 | CuOTf·0.5C6H6, 15% | 100 | 38% |
| 3 | Cu(OTf)2, 15% | 100 | 25% |
| 4 | Cu(MeCN)4PF6, 15% | 100 | 50% |
| 5 c | Cu(MeCN)4PF6, 15% | 120 | 96% |
| 6 c | Cu(MeCN)4PF6, 15% | 130 | 99% |
| 7 d , e | Cu(MeCN)4PF6, 15% | 130 | 99% |
| 8 | No catalyst | 100 | N.R. |
| 9 | Rh2(hfb)4, 1% | 100 | N.R. f |
aTriazole (1 equiv.), alkyne (3 equiv.), Cu cat. (15 mol%), toluene (1 M) in a Wheaton V-vial capped with a Mininert syringe valve.
bGC/MS yields are given.
c1.2 equiv. of alkyne was used.
dIn air with 1.2 equiv. of alkyne.
eLower catalyst loading led to decreased reaction yields.11
fPolymerization of the alkyne was observed; hfb = heptafluorobutyrate.
