Fig. 7.
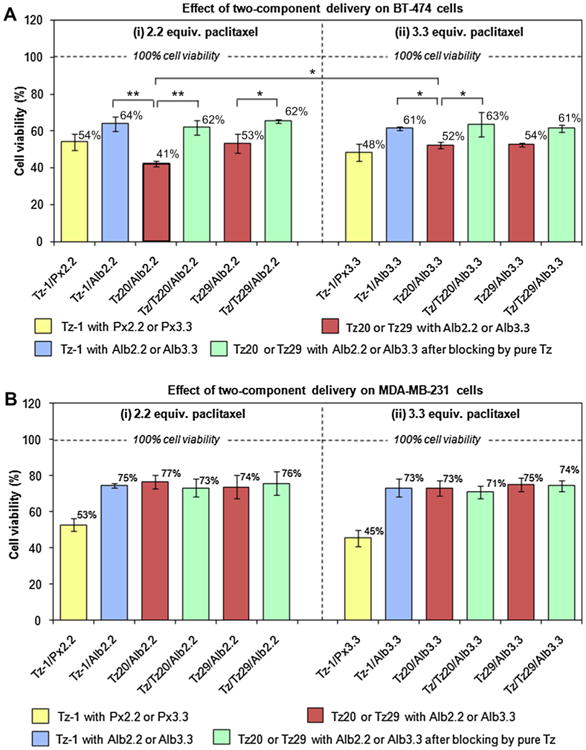
Therapeutic effect of two-component delivery strategy on (A) HER2-positive BT-474 cells and (B) HER2-negative MDA-MB-231 cells. Cells were treated with trastuzumab-based pretargeting components (Tz-1, Tz20, or Tz29), followed by paclitaxel-containing therapeutic components ((i) for Px2.2 or Alb2.2 and (ii) for Px3.3 or Alb3.3). Receptor blocking with unlabeled trastuzumab was also performed prior to the pretargeting step to verify the specific HER2 receptor-mediated targeting. Cell viability was measured using WST-8 cell viability kit. The changes in cell viability were considered significant when p-values were lower than 0.05 (*p < 0.05, **p < 0.01).
