Abstract
Background: There are inconsistencies in findings as to whether cannabis use has a negative impact on clinical outcomes for people with established psychosis. Effects may be more evident on patients with recent onset psychosis. Aim: To investigate the relationship between cannabis use and clinical outcome, including whether change in cannabis use affects psychotic symptoms, affective symptoms, functioning and psychotic relapse in a sample of people in early psychosis with comorbid cannabis abuse or dependence. Methods: One hundred and ten participants were examined prospectively with repeated measures of substance use antecedent to psychopathology at baseline, 4.5, 9, and 18 months. We used random intercept models to estimate the effects of cannabis dose on subsequent clinical outcomes and whether change in cannabis use was associated with change in outcomes. Results: There was no evidence of a specific association between cannabis use and positive symptoms, or negative symptoms, relapse or hospital admissions. However, a greater dose of cannabis was associated with subsequent higher depression and anxiety. Change in the amount of cannabis used was associated with statistically significant corresponding change in anxiety scores, but not depression. Additionally, reductions in cannabis exposure were related to improved patient functioning. Conclusions: Reducing cannabis may be directly associated with improvements in anxiety and functioning, but not other specific symptoms.
Key words: psychosis, cannabis, substance use, dual diagnosis
Introduction
People with a schizophrenia diagnosis have higher rates of cannabis use than the general population: a meta-analysis of 35 studies from 16 countries reports a median lifetime rate of cannabis use disorders of 27.1% compared with the general population rate of only 8%.1 The possible detrimental effect of such widespread drug use in this population has been a major focus of research interest. There is now consistent evidence that cannabis is an independent risk factor for the development of psychosis,2 and that cannabis use is associated with earlier onset of psychotic illness,3 but the findings as to whether cannabis exposure has a negative impact on clinical outcomes for those who have already developed a psychosis are less in agreement. To examine causative effects, the systematic review of Zammit and colleagues4 focused exclusively on studies with longitudinal designs and where cannabis consumption was assessed prior to the timing of the clinical outcomes. Findings indicated that the most consistent links were between cannabis use and psychotic relapse,5–8 with 2 studies also showing that cannabis use was associated with an increase in hospitalizations.7,9 However, evidence for associations between cannabis and psychotic or other specific symptoms is less robust. Three studies included in the Zammit et al review6,10,11 and a further 3 with longitudinal designs since published12–14 found reductions in cannabis status/use were associated with improved positive psychotic symptoms, while 2 studies from the review7,15 and a further 3 recent studies16–18 failed to find associations.
The methodological issues that may contribute to these inconsistent findings have previously been noted.4,16 Most critically, many studies did not adjust for potential confounds known to be associated both with cannabis use and poorer clinical outcome, such as gender, sociodemographics, and medication adherence4; nor did they take account of other illicit drug use and alcohol consumption and baseline illness severity. In an earlier study of people with established psychosis,16 we controlled for these factors, employing a longitudinal design with time-lagged measures of psychopathology, use of cannabis and other substances, and adjusted for a wide range of confounds.16 Additionally, we examined the frequency and amount of cannabis consumed in relation to outcomes, since if there is a relationship between cannabis and psychotic symptoms, it is likely to be dose related. Moreover, by covarying previous cannabis use and previous symptoms, we were able to examine whether change in cannabis use predicted change in outcomes. We found that cannabis use had no effect on positive or negative or general psychotic symptoms, hospital admissions or relapses. We did find that greater cannabis exposure was associated with worse functioning, albeit with a small effect size.
The participants in our previous study were people with long established psychosis in contrast with the young, recent onset groups seen in the majority of studies cited above. We suggest that cannabis might have had a detrimental effect in the early stages of the illness but that further increases in consumption have no detectable impact, with consequences no longer reversible and/or ceiling effects operating such that further increases in consumption are not evident. A recent meta-analysis found that giving up substance use was associated with significant improvement in symptoms and disability in early stage psychosis but not for later stages.19 Hence the current study employs an 18-month longitudinal design and aims to examine the possibility that cannabis has an impact on clinical outcomes in young people with recent onset psychosis. We attempt to address the shortcomings of previous studies by not only examining cannabis dose, but also assessing the type of cannabis used to give a better indication of consumption of ∆-9-tetrahydrocannabinol (THC), the primary psychoactive ingredient of cannabis.20 Higher potency cannabis types containing higher levels of THC and lower cannabidiol (sinsemilla, “skunk”) are associated with an increased risk of psychosis and an earlier psychosis onset.21,22 Furthermore, in addition to examining the impact of cannabis on psychotic symptoms, relapse, and functioning, we also examine its relationship to affective symptoms. In nonpsychotic samples, there is now increasing evidence that regular cannabis use is associated with anxiety23 and may be associated with an increased risk for developing depressive disorders.24 While to date, 3 prospective studies of people with psychosis11,14,18 have found cannabis misuse was not associated with change in depression scores, the relationship of cannabis use to anxiety is less clear.
In summary, we examined the longitudinal relationships of cannabis use to a range of clinical outcomes in a large sample of people who were within 3 years of a psychosis diagnosis. We tested the specific hypotheses that greater cannabis dose (taking into account the estimate of THC content) would be associated with higher positive and anxiety symptoms; and that reduction in cannabis dose would be associated with reduction in these symptoms, after adjusting for potential confounds and antecedent symptom severity.
Methods
Participants and Design
One hundred and ten participants who met diagnostic criteria for nonaffective psychotic disorder with comorbid cannabis abuse or dependence (DSM IV) were recruited from 5 Early Intervention Services in the North West of England, UK. Participants were initially recruited as part of a randomized controlled single blind trial of a psychological intervention25 where participants were allocated to receive either brief (12 session) motivational interviewing with cognitive behavioral therapy (MICBT, n = 38); long (24 session) MICBT (n = 37), or treatment as usual (n = 35). The current study reports secondary analyses of the data obtained from that trial. Additional inclusion criteria were as follows: aged 16–35; history of cannabis use of at least 1 day per week in at least half the weeks in the 3 months preceding assessment; having stable accommodation (not street homeless or roofless); possessing sufficient English to reliably complete assessments; no significant history of organic factors implicated in the etiology of psychotic symptoms and able to give informed consent.
After a complete description of the study, written informed consent was obtained by trained research assistants. Diagnostic and substance use eligibility was confirmed using the Structured Clinical Interview for DSM IV Axis 1 disorders (SCID).26 A substance use checklist was used to determine frequency and amount of substance use, including alcohol. After screening, participants completed baseline assessment measures. Follow-up assessments were conducted at 4.5, 9, and 18 months and were conducted by trained assessors who were blind to treatment allocation. Ethical approval was granted by the North West Lancaster Research Ethics Committee (08/H1015/82).
Measures
Cannabis Use.
Frequency (percentage days used) and average daily weight per cannabis using day (grams) was assessed for the 3 months preceding baseline, 4.5-, 9-, and 18-month follow-up assessments using the Timeline Followback (TLFB) method.27 At each assessment, participants were asked to report all cannabis use that had taken place during the previous 90 days. The TLFB has good reliability and validity in dual diagnosis populations,28,29 including in the current sample where hair analysis showed good agreement with self-reports.25 Prior to detailing cannabis use, participants were asked to state the type of cannabis used most frequently, describing its appearance (color and texture) and strength. Responses were categorized into high potency cannabis (skunk variants) or low potency (resin and hashish) based on potency data obtained from the UK Home Office Potency Study.30 Participants were also asked to detail route of consumption (eg, “joints” or “bongs”) and to estimate how much cannabis (in grams) went into each receptacle. For participants whose use was described in joints and who could not provide an estimate of grams used we used the average amount for the sample: 0.3 g per joint. We took into account the potency of the cannabis smoked by multiplying the weight of the more potent types by 1.5 since the average THC content of skunk/sinsemilla found in the UK Home Office Potency Study was 15%, compared with 5% for resin.
Other Substances.
Frequency of use of all substances (percentage days used) was also calculated from the TLFB. Participants reported units of alcohol consumed in the 90 days prior to assessment and recounted consumption of other illicit substances. Two binary variables were created from these data and entered as time-dependent covariates (baseline, 4.5, 9, and 18 months) to adjust for use of substances other than cannabis: alcohol use above safe limits (exceeding 4 UK standard units on average per drinking day for men; 3 for women (1 unit = 10ml pure ethanol) and any other illicit drug use.
Clinical Assessments.
The Positive and Negative Syndrome Scales (PANSS)31 were used to assess positive, negative, and general psychopathology symptoms in the previous week. Global functioning was assessed using the Global Assessment of Functioning Scale (GAF).32 Anxiety was assessed using the Beck Anxiety Inventory (BAI)33 and Depression using the Calgary Depression Scale for Schizophrenia (CDSS).34
Data on relapse and hospitalization was obtained from psychiatric case notes. Frequency and duration of relapses and hospitalization in the 9 months before baseline and during the 18-month intervention and follow-up period was recorded. Relapse was defined as an exacerbation of psychotic symptoms that lasted for longer than 2 weeks and resulted in a change in patient management (increased observation by the clinical team; increase in antipsychotic medication or both). The start and end date of each relapse was recorded along with verbatim extractions from the notes detailing changes in symptoms and clinical management. Start and end date of all hospital admissions for psychiatric purposes were also recorded. Admissions for preplanned changes in medication were not included.
Case note review was also used to determine antipsychotic medication at baseline (type and dose) and this information was transformed into chlorpromazine equivalents in milligrams.
Assessors rated 10 “gold standard” video-recorded PANSS interviews before conducting trial assessments. Mean intraclass correlations coefficients (ICCs) indicated excellent interrater reliability: positive subscale 0.87; negative 0.86; general 0.87; total 0.89; and for the GAF: 0.94. Ratings were monitored throughout the trial and ICCs remained high: positive subscale 0.90; negative 0.85; general 0.90; total 0.95. Assessors extracted hospitalization and relapse data from 6 test cases prior to accessing participant case notes. Interrater reliability across assessors was excellent with 100% agreement on admission (yes/no); number of admissions and numbers of weeks in admission. ICCs for relapse variables were also high with 0.86 obtained for relapse (yes/no) and 0.97 for number of relapses.
Additional Covariate Assessments.
Demographic information (age, gender, living arrangements, ethnicity, education, employment, socioeconomic status) was collected at baseline via self-report. Additionally, the Drug Attitude Inventory (DAI) was used as a proxy measure for compliance with prescribed antipsychotic medication.35 The DAI has been criticized on validity grounds, but does correlate well with a more recent self-report compliance measure.36
Data Analysis
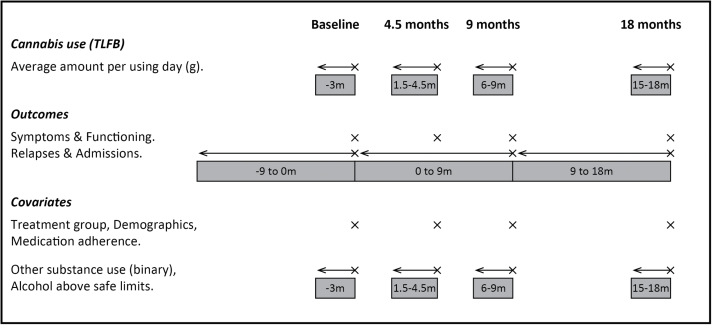
All statistical analyses used Stata version 13.0 using the “xtlogit” or “xtreg” commands with mle option. We used the measurement of cannabis consumption in the previous 90 days (grams per cannabis using day) derived from the TLFB (figure 1) to estimate the effect of cannabis use on subsequent clinical outcomes for symptoms and functioning.
Fig. 1.
Repeated assessment of cannabis use, outcomes, and covariates. Adapted from Barrowclough et al. 16
Random intercept models were used to produce an average estimate of the linear correlation between cannabis use and each outcome across all 4 time periods (baseline, 4.5, 9, and 18 months) taking the within-subject correlation into account. For relapses and hospital admissions (yes/no between 0–9 months, and 9–18 months), the effect of cannabis use in the 90 days prior to the start of the relevant time period was examined, and random intercept logistic models were used to combine the effects observed at both time points.
For the analysis to assess whether change in cannabis use was associated with change in symptoms, we excluded the measures at 4.5 months and calculated change scores between baseline and 9 months, and 9 and 18 months so that the change was over a constant period. Change in outcome was then included as the dependent variable in the random intercept models, with change in cannabis use as the explanatory variable.
All analyses were performed first without adjusting for covariates and then with adjustment for the following set of baseline and time-varying covariates: treatment group, age, gender, stable living, ethnicity, higher education, employment, socioeconomic status, drug attitude inventory, alcohol use above safe levels (at each time point), any other substance use (at each time point). In addition, when considering relapse and admissions as outcomes, we adjusted for baseline measures of these.
Random intercept models were used because cannabis use and the clinical outcomes were repeated measures and therefore not independent within each person. The regression coefficients from these models can be interpreted as in a standard linear or logistic regression, where having a 1 unit increase in the cannabis use variables is associated on average with having higher or lower outcomes corresponding to the coefficients reported in the tables below. Table 1 shows how a 1 g increase (approximately 3 joints) in the average daily amount of cannabis is associated on average with having higher or lower outcomes corresponding to the coefficients reported.
Table 1.
Association Between Cannabis Use and Clinical Outcomes Over Time
| Dependent Variable | Baseline | 4.5 Months | 9 Months | 18 Months | Coefficient of Cannabis Measure for Average Daily Weight (g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | Unadjusted (95% CI); N (obs)a | Adjusted (95% CI); N (obs)a | |
| PANSS positive | 110 | 15.03 (4.16) | 72 | 14.04 (4.34) | 70 | 13.77 (4.33) | 71 | 13.32 (4.56) | 0.06 (−0.18, 0.30); 110 (319) | 0.01 (−0.24, 0.25); 102 (302) |
| PANSS negative | 110 | 14.08 (4.78) | 72 | 13.51 (5.15) | 70 | 13.2 (4.40) | 70 | 14.36 (4.95) | 0.24 (0.00, 0.49); 110 (319)* | 0.22 (−0.03, 0.46); 102 (302) |
| PANSS general | 110 | 34.11 (7.07) | 72 | 31.90 (8.13) | 70 | 32.43 (8.12) | 70 | 33.39 (8.61) | 0.52 (0.08, 0.96); 110 (319)* | 0.47 (0.03, 0.91); 102 (302)* |
| PANSS delusions | 110 | 3.19 (1.51) | 72 | 3.07 (1.48) | 70 | 2.86 (1.40) | 71 | 2.76 (1.43) | −0.00 (−0.09, 0.08); 110 (319) | −0.03 (−0.11, 0.06); 102 (302) |
| PANSS hallucinations | 110 | 2.6 (1.60) | 72 | 2.58 (1.59) | 70 | 2.81 (1.68) | 71 | 2.45 (1.48) | 0.06 (−0.03, 0.15); 110 (319) | 0.07 (−0.02, 0.16); 102 (302) |
| Depression | 110 | 6.95 (4.74) | 71 | 5.86 (4.27) | 71 | 6.04 (4.61) | 70 | 6.71 (5.31) | 0.29 (0.02, 0.57); 110 (319)* | 0.31 (0.04, 0.57); 102 (302)* |
| Anxiety | 110 | 18.05 (13.64) | 75 | 15.62 (12.41) | 73 | 14.85 (12.27) | 69 | 15.30 (13.56) | 0.91 (0.20, 1.63); 110 (325)* | 0.94 (0.24, 1.65); 102 (306)* |
| Functioning | 110 | 37.27 (9.04) | 72 | 39.25 (9.62) | 71 | 40.39 (12.85) | 70 | 41.93 (15.11) | −0.58 (−1.24, 0.08); 110 (319) | −0.40 (−1.05, 0.25); 102 (302) |
| N (%) | N (%) | OR (95% CI); N (obs)a | OR (95% CI); N (obs)a | |||||||
| Relapse (yes) (past 9 months) | — | — | — | — | 109 | 27 (24.8) | 107 | 15 (13.6) | 0.96 (0.78, 1.18); 109 (187) | 1.00 (0.76, 1.32); 101 (174) |
| Admissions (yes) (past 9 months) | — | — | — | — | 108 | 13 (12.0) | 107 | 13 (12.1) | OR = 0.79 (0.54, 1.17); 108 (186) | OR = 0.76 (0.46, 1.25); 100 (173) |
Note: PANSS, Positive and Negative Syndrome Scale.
a N refers to the number of participants included in the model, obs refers to the number of observations included in the model (repeated within participant).
*P < .05.
Using maximum likelihood as our estimation approach uses all available data, which allows for nonmonotonic missingness under a missing at random assumption, conditional on the variables in the model. The total number of observations in the model becomes the total number of observed outcomes and predictors on all participants at each study assessment point and so varies according to outcome being analyzed. In addition, the adjusted analysis has fewer observations when there is also additional missingness in the independent variables. The number of participants and observations included in the unadjusted and adjusted models are shown with the results in tables 1 and 3.
Table 3.
Association Between Change in Cannabis Use and Change in Clinical Outcomes
| Dependent Variable | Coefficient of Cannabis Measure for Average Daily Weight (g) | |
|---|---|---|
| Unadjusted (95% CI); N (obs)a | Adjusted (95% CI); N (obs)a | |
| PANSS positive | −0.09 (−0.43, 0.25); 69 (130) | −0.12 (−0.45, 0.22); 65 (123) |
| PANSS negative | 0.31 (−0.02, 0.64); 69 (130) | 0.28 (−0.04, 0.61); 65 (123) |
| PANSS general | 0.46 (−0.21, 1.13); 69 (130) | 0.54 (−0.12, 1.19); 65 (123) |
| PANSS delusions | −0.08 (−0.21, 0.05); 69 (130) | −0.10 (−0.23, 0.04); 65 (123) |
| PANSS hallucinations | 0.04 (−0.08, 0.16); 69 (130) | 0.05 (−0.07, 0.18); 65 (123) |
| Depression | −0.06 (−0.48, 0.37); 70 (132) | 0.09 (−0.32, 0.51); 66 (125) |
| Anxiety | 1.07 (−0.03, 2.16); 73 (135) | 1.27 (0.12, 2.42); 68 (127)* |
| Functioning | −1.01 (−2.07, 0.06); 70 (131) | −1.29 (−2.33, −0.23); 66 (124)* |
Note: PANSS, Positive and Negative Syndrome Scale.
a N refers to the number of participants included in the model, obs refers to the number of observations included in the model (repeated within participant).
*P < .05.
Results
Participants
A total of 110 participants were included in the analyses. Data on cannabis use was collected for 83 participants (75.5%) at 4.5 months, 79 (71.8%) at 9 months, and 75 (68.2%) at 18 months. Table 2 presents demographic, psychiatric, and substance use information obtained from participants at baseline. Participants were largely male, aged in their mid-20s and unemployed. Mean history of psychosis was less than 18 months and history of cannabis use was approximately 10 years. The majority met criteria for cannabis use dependence. Eighteen were using alcohol above safe limits (with 10 of these meeting abuse or dependence criteria according to the SCID) and 20 were using other substances in addition to cannabis and alcohol (namely cocaine; stimulants; sedatives; and hallucinogens).
Table 2.
Demographic, Psychiatric History, and Baseline Substance Use Variables
| N (%) | |
|---|---|
| Age in years, mean (SD) | 24.2 (5.0) |
| Gender: male | 98 (89.1) |
| Living arrangements | |
| Alone/house-share/hostel | 44 (40.0) |
| With partner or family | 66 (60.0) |
| Ethnicity | |
| White | 102 (92.7) |
| Black and minority ethnic | 8 (7.3) |
| Attended higher education | 56 (50.9) |
| Employment | |
| Unemployed/retired | 89 (80.9) |
| Employed/self-employed | 8 (7.3) |
| Student | 13 (11.8) |
| History of psychosis (months), median (range) | 15.4 (1.4–59.8) |
| Duration of untreated psychosis <4 months | 40 (36.4) |
| Antipsychotic medication (CPZ equivalence), median (range) | 200 (0–800) |
| Compliant with medication (DAI) | 85 (77.3) |
| Baseline diagnosis (SCID-I) | |
| Schizophrenia | 54 (49.1) |
| Schizophreniform | 9 (8.2) |
| Schizoaffective | 13 (11.8) |
| Delusional disorder | 9 (8.2) |
| Substance-induced psychosis | 6 (5.5) |
| Psychotic disorder not otherwise specified | 19 (17.3) |
| PANSS, mean (SD) | |
| Positive | 15.0 (4.2) |
| Negative | 14.1 (4.8) |
| General | 34.1 (7.1) |
| Depression (CDS), mean (SD) | 7.0 (4.7) |
| Anxiety (BAI), mean (SD) | 17.4 (11.9) |
| Global functioning (GAF), mean (SD) | 37.3 (9.0) |
| Relapsed (9 months pre-baseline) | 51 (46.4) |
| Admitted (9 months pre-baseline) | 23 (20.9) |
| History of cannabis use (years), mean (SD) | 10.0 (5.1) |
| Substance use disorder (SCID-I) | |
| Cannabis abuse | 10 (9.1) |
| Cannabis dependence | 100 (90.9) |
| Alcohol abuse | 7 (6.4) |
| Alcohol dependence | 3 (2.7) |
| Substance use, mean (SD) | |
| Percentage days cannabis use in last 90 | 65.2 (30.5) |
| Average amount of cannabis used per using day (grams), uncorrected | 1.7 (1.5) |
| Percentage days of use of all substances | 71.8 (27.0) |
| Alcohol use above safe limits | 18 (16.4) |
| Polysubstance use (use of substances other than alcohol or cannabis) | 20 (18.2) |
Note: CPZ, chlorpromazine; DAI, Drug Attitude Inventory; SCID, Structured Clinical Interview for DSM IV Axis 1; PANSS, Positive and Negative Syndrome Scale; CDS, Calgary Depression Scale; BAI, Beck Anxiety Inventory; GAF, Global Assessment of Functioning Scale.
Amount and Stability of Cannabis Use
The mean frequency of cannabis use was 65% of days in the preceding 90 days (SD 30%) equating to 4–5 days use per week. Average amount of cannabis used per using day (uncorrected) was 1.3 g, equating to approximately 4 joints per day. Corrected for type of cannabis, average daily use was 2.3 g, equivalent to 7–8 “standard” joints per day. Reductions in average daily cannabis consumed were found in 55.4% (46/83) of participants at 4.5 months follow-up, 59.5% at 9 months (47/79), and 63.2% at 18 months (48/76).
Is Cannabis Use Related to Clinical Outcomes?
Average daily weight of cannabis use was associated with PANSS negative and general symptoms, depression, and anxiety in the unadjusted analyses such that greater amounts of cannabis use were associated with more severe symptoms. After adjustment for covariates, relationships between cannabis dose and PANSS general symptoms, depression and anxiety remained (table 1). There were no significant associations between cannabis use and positive symptoms; functioning; relapse; or admission.
Does Change in Cannabis Use Predict Change in Clinical Outcomes?
Change in cannabis use did not significantly predict change in PANSS symptom measures in either the unadjusted or adjusted analyses (table 3). In the adjusted but not the unadjusted analyses, changes in anxiety and functioning were associated with changes in cannabis use: an increase in cannabis use predicted greater anxiety and poorer functioning.
We additionally explored whether abstinence from cannabis was associated with change in clinical outcomes by examining whether there was a significant difference between the abstinent group and the still using group by including this variable as the cannabis use variable in the above models. There were 24 occasions when participants were abstinent (20 participants) for the 90 days preceding clinical assessment. Abstinence from cannabis on these occasions was related to improved global functioning in both the unadjusted and adjusted analyses (adjusted coefficient = 4.95, 95% CI = 0.46–9.44, P < .05).
Discussion
Contrary to our prediction, in this sample of people with recent onset psychosis, we found no evidence of a specific association between cannabis use and positive symptoms. The analyses took account of an estimate of THC content consumed and we did find associations between a greater amount of cannabis used and more affective symptoms for both observer rated affective symptoms (PANSS general symptoms) and self-reports of both depression and anxiety. There was a significant relationship between dose of cannabis and subsequent severity of these symptoms over the 18-month period of study. Increase in the amount of cannabis used was associated with a statistically significant increase in anxiety scores, but not depression. We also found that reductions in cannabis exposure were associated with increased patient functioning. There were no relationships between cannabis use and relapse or admissions, and the positive association between negative symptoms and cannabis was not evident once covariates were taken into account.
Our finding that cannabis dose was not related to positive symptoms is consistent with the results of our previous study,16 which used the same analyses strategies but was conducted with a sample of somewhat older psychosis patients (means 35 vs 24) who had a history of psychosis of over 10 years. In the current sample of younger patients, the length of illness was typically just over 1 year, but inspection of the profiles of the participants in the 2 studies indicates that there were few differences in terms of symptomatic, substance use, or other demographic variables. Thus, our findings do not provide support for the contention that cannabis might have a more detrimental effect on positive symptoms in the early stages of the illness. Six previous longitudinal studies with time-lagged designs and recent onset samples found changes in cannabis status/use associated with positive psychotic symptoms6,10–14 and 3 failed to find associations.7,15,17 Two further studies including people with more established illness16,18 showed no association between cannabis use and severity of psychotic symptoms. We have previously noted that methodological differences may be responsible for the inconsistencies in findings in this area, including failure to adequately examine the impact of dose and type of cannabis, the limited adjustment of potential confounds in some studies, and differences in statistical methodologies.16 Moreover, the sample of cannabis users in our current study was larger than the majority of previous studies, and for previous larger sample studies, only 2 assessment points were included.12,13 It should be noted, that while our results do not demonstrate any ongoing associations between cannabis and positive symptoms, they do not rule out the possibility that cannabis had already had a negative impact on these symptoms, either directly or indirectly, with irreversible consequences. In contrast to the negative findings regarding positive symptoms, our study found that there were associations between the absolute levels of cannabis consumed and both anxiety and depression symptoms over the 18 months of the study. Using the measurement lag in the continuous cannabis measure to predict symptom severity at each time point and averaging the effects over the 3 assessment time points, we found that when cannabis levels were higher, people were experiencing subsequent higher affective symptoms. While in non-psychotic samples, there is now increasing evidence that regular cannabis use is associated with depression,24,37–39 3 previous studies that have examined longitudinal relationships between cannabis use and depression in people with psychosis had negative findings.11,14,18 One study18 had a relatively small sample of cannabis users (n = 68); one14 used a simple binary classification of cannabis use in participants (users vs nonusers) to predict future symptoms using varying time intervals; and another11 did not account for the dose of cannabis. Moreover, none of the studies accounted for the difference in potencies of cannabis type. Hence, we would argue that our results regarding the association of cannabis use with depression are more valid than the findings of previous studies. The change analysis indicated that reducing cannabis is unlikely to lead to an improvement in depressive symptoms, and this is consistent with current evidence of links between cannabis and mood. There is little research evidence supporting the biological hypothesis that cannabis use causes changes in neurotransmitter systems that are linked to depressed mood, and better support for the effects of cannabis on mood being socially mediated by a complex range of factors.37 Hence, reduction in cannabis alone would be unlikely to resolve depressed mood in people with psychosis, who often face many social problems. On the other hand, although the relationship between cannabis and anxiety is complex and dose and type related, THC induces anxiety.23 Thus, when controlling for consumption of THC, we are more likely to see reduction in cannabis associated with an improvement in anxiety symptoms, as seen in this study.
Cannabis use was associated with an improvement in general functioning, a finding that was also evident in our earlier study with a sample of patients with longer illness history.16 The GAF is a measure of overall psychosocial functioning. We have previously suggested that the association of cannabis use with this general functioning measure may indicate that the impacts of cannabis on people with psychosis are quite complex and variable. While not all the cannabis-related effects are detectable in specific symptom measures when examining population-level effects, cannabis may contribute to poorer functioning by a variety of mechanisms at the individual level.
One major advantage of the current study is that we were able to take account of the differing THC content in the types of cannabis participants reported. We were also able to analyze both the weight and frequency of cannabis used and had measures which supported the validity of the self-reports of consumption. Hence, we were able to take account of cannabis dose much more accurately than previous studies. Additionally, as previously emphasized, our study took account of a wide range of potential confounds and conducted both associative and change time-lagged analyses. In terms of limitations, we note that we examined only the relatively long-term and durable consequences of cannabis use. The transient impacts of cannabis on both psychotic symptoms and anxiety have been demonstrated in experimental studies.40,41 Our sample size of cannabis users was relatively large compared with previous studies, but may have had insufficient power to detect significant effects on clinical outcomes from drug use where these were small. The calculation of the THC content was only a best estimate, gauged from self-report of both the amount and type of cannabis consumed. Future research should seek to determine the exact content of the cannabis consumed.
In conclusion, the findings from this methodologically robust study indicate that cannabis has no direct impact on the positive symptoms of psychosis in the early phase of psychosis. However, during this recent onset period, greater exposure to cannabis is associated with higher levels of depression, possibly mediated by social factors; and reducing cannabis may lead to direct improvements in anxiety symptoms and better functioning. Future studies with larger samples are required to replicate and take forward this clinically important area of study.
Funding
This article represents research commissioned by the UK’s National Institute for Health Research (NIHR) under its Programme Grants for Applied Research scheme (RP-PG-0606-1302).
Acknowledgment
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Koskinen J, Löhönen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36:1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. [DOI] [PubMed] [Google Scholar]
- 3. Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. [DOI] [PubMed] [Google Scholar]
- 4. Zammit S, Moore TH, Lingford-Hughes A, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193:357–363. [DOI] [PubMed] [Google Scholar]
- 5. Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD. Substance misuse in first-episode psychosis: 15-month prospective follow-up study. Br J Psychiatry. 2006;189:229–234. [DOI] [PubMed] [Google Scholar]
- 6. Hides L, Dawe S, Kavanagh DJ, Young RM. Psychotic symptom and cannabis relapse in recent-onset psychosis. Prospective study. Br J Psychiatry. 2006;189:137–143. [DOI] [PubMed] [Google Scholar]
- 7. Horcajadas A, Romero S, Calo J. Relevance of drug use in clinical manifestations of schizophrenia [Spanish]. Actas Esp Psiquiatr. 2002;30:65–73. [PubMed] [Google Scholar]
- 8. Martinez-Arevalo MJ, Calcedo-Ordoñez A, Varo-Prieto JR. Cannabis consumption as a prognostic factor in schizophrenia. Br J Psychiatry. 1994;164:679–681. [DOI] [PubMed] [Google Scholar]
- 9. Caspari D. Cannabis and schizophrenia: results of a follow-up study. Eur Arch Psychiatry Clin Neurosci. 1999;249:45–49. [DOI] [PubMed] [Google Scholar]
- 10. Grech A, Van Os J, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. Eur Psychiatry. 2005;20:349–353. [DOI] [PubMed] [Google Scholar]
- 11. Degenhardt L, Tennant C, Gilmour S, et al. The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychol Med. 2007;37:927–934. [DOI] [PubMed] [Google Scholar]
- 12. Clausen L, Hjorthøj CR, Thorup A, et al. Change in cannabis use, clinical symptoms and social functioning among patients with first-episode psychosis: a 5-year follow-up study of patients in the OPUS trial. Psychol Med. 2014;44:117–126. [DOI] [PubMed] [Google Scholar]
- 13. Stone JM, Fisher HL, Major B, et al. ; MiData Consortium. Cannabis use and first-episode psychosis: relationship with manic and psychotic symptoms, and with age at presentation. Psychol Med. 2014;44:499–506. [DOI] [PubMed] [Google Scholar]
- 14. Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr Res. 2005;75:135–137. [DOI] [PubMed] [Google Scholar]
- 16. Barrowclough C, Emsley R, Eisner E, Beardmore R, Wykes T. Does change in cannabis use in established psychosis affect clinical outcome? Schizophr Bull. 2013;39:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faber G, Smid HG, Van Gool AR, Wunderink L, van den Bosch RJ, Wiersma D. Continued cannabis use and outcome in first-episode psychosis: data from a randomized, open-label, controlled trial. J Clin Psychiatry. 2012;73:632–638. [DOI] [PubMed] [Google Scholar]
- 18. van Dijk D, Koeter MW, Hijman R, Kahn RS, van den Brink W. Effect of cannabis use on the course of schizophrenia in male patients: a prospective cohort study. Schizophr Res. 2012;137:50–57. [DOI] [PubMed] [Google Scholar]
- 19. Mullin K, Gupta P, Compton MT, Nielssen O, Harris A, Large M. Does giving up substance use work for patients with psychosis? A systematic meta-analysis. Aust N Z J Psychiatry. 2012;46:826–839. [DOI] [PubMed] [Google Scholar]
- 20. Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 21. Di Forti M, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in Cannabis Users [published online ahead of print December 17, 2013]. Schizophr Bull. 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515–523. [DOI] [PubMed] [Google Scholar]
- 24. Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2013:1–14. [DOI] [PubMed] [Google Scholar]
- 25. Barrowclough C, Marshall M, Gregg L, Fitzsimmons M, Tomenson B, Warburton J, Lobban F. A phase specific psychological therapy for people with problematic cannabis use following a first episode of psychosis: A Randomised Controlled Trial. Psychol Med. In press. [DOI] [PubMed] [Google Scholar]
- 26. First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV). American Psychiatric Press; 1997. [Google Scholar]
- 27. Norberg MM, Mackenzie J, Copeland J. Quantifying cannabis use with the timeline followback approach: a psychometric evaluation. Drug Alcohol Depend. 2012;121:247–252. [DOI] [PubMed] [Google Scholar]
- 28. Barrowclough C, Haddock G, Wykes T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ. 2010;341:c6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hjorthoj CR, Fohlmann A, Larsen AM, Gluud C, Arendt M, Nordentoft M. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: the CapOpus randomized trial. Psychol Med. 2012:1–12. [DOI] [PubMed] [Google Scholar]
- 30. Hardwick S, King LA. Home Office Cannabis Potency Study 2008. St Albans: Home Office Scientific Development Branch; 2008. [Google Scholar]
- 31. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 32. APA. Diagnostic and Statistical Manual for Mental Disorders. 4th ed. Washington, DC: American psychiatric Association; 1994. [Google Scholar]
- 33. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. [DOI] [PubMed] [Google Scholar]
- 34. Addington D, Addington J, Matickatyndale E. Assessing Depression in Schizophrenia - the Calgary Depression Scale. Brit J Psychiat. 1993;163:39–44. [PubMed] [Google Scholar]
- 35. Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13:177–183. [DOI] [PubMed] [Google Scholar]
- 36. Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr Res. 2000;42:241–247. [DOI] [PubMed] [Google Scholar]
- 37. Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–1504. [DOI] [PubMed] [Google Scholar]
- 38. Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325:1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Laar M, van Dorsselaer S, Monshouwer K, de Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. 2007;102:1251–1260. [DOI] [PubMed] [Google Scholar]
- 40. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. [DOI] [PubMed] [Google Scholar]
- 41. D’Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. [DOI] [PubMed] [Google Scholar]