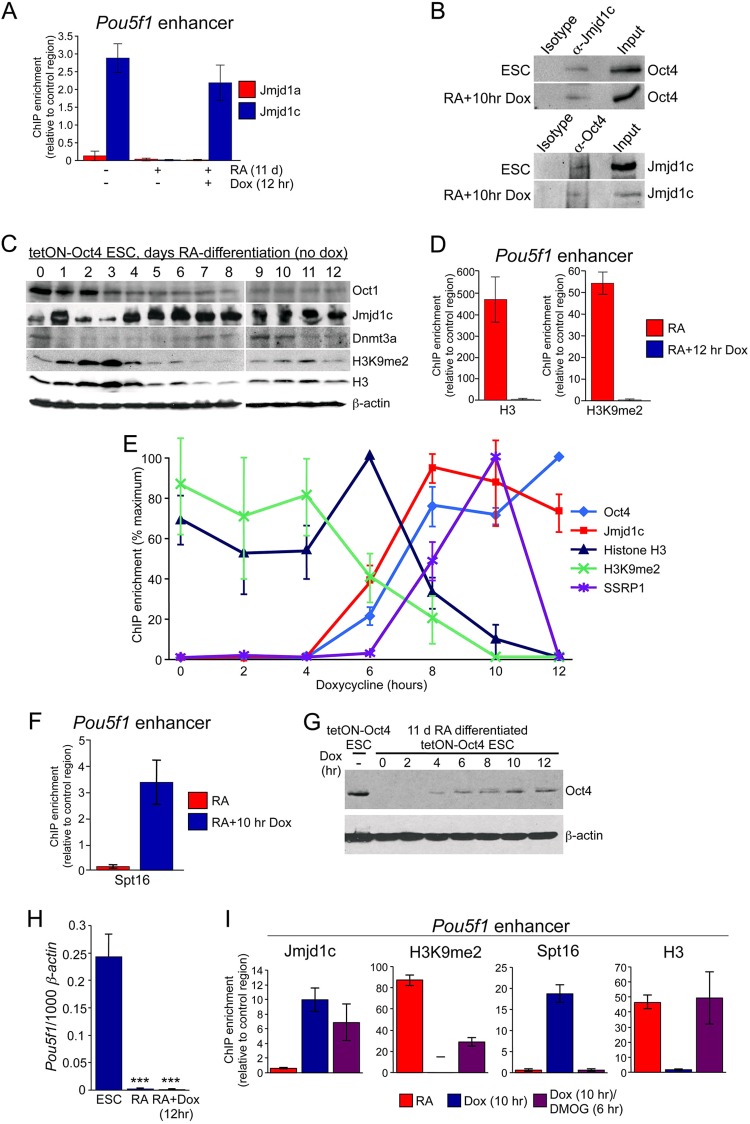
FIG 3.
Oct4 associates with Jmjd1c and FACT at Pou5f1. (A) Jmjd1a and Jmjd1c ChIP enrichment was measured at the Pou5f1 enhancer in tetON-Oct4 ESCs, ESCs undergoing RA-mediated differentiation for 11 days, and similarly differentiated ESCs treated with doxycycline for 12 h to induce Oct4. (B) Coimmunoprecipitation assay using undifferentiated tetON-Oct4 ESCs (without doxycycline in the medium). Lysates were immunoprecipitated with anti-Jmjd1c antibodies or with an isotype control, followed by Western blotting with Oct4 antibodies. (C) Differentiation time course of Oct1, Jmjd1c, Dmnt3a, and H3K9me2 protein expression (without doxycycline). Western blot data are shown. Histone H3 and β-actin were used as loading controls. (D) Histone H3 and H3K9me2 ChIP enrichment in 11-day-differentiated ESCs (with or without 12 h of doxycycline treatment to induce ectopic Oct4 expression). (E) A ChIP time course was performed using ESCs differentiated for 11 days with RA and subsequently induced using doxycycline for the indicated times. ChIP was performed at the Pou5f1 enhancer with the indicated antibodies. For each antibody, the ChIP signal is shown as a percentage of the maximum (100%). Each experiment was performed in biological triplicates. Error bars depict the standard deviations. In cases where the same time point showed maximum signal for all biological and technical replicates (Oct4, histone H3, and SSRP1), the signal was 100%, and the standard deviation for that time point was 0. In other cases (Jmjd1c and H3K9me2) the maximum signal was at different times for different replicates, and an error was derived at all time points. (F) A similar ChIP experiment was performed at the 0- and 10-h time points with antibodies against the FACT subunit Spt16. (G) A Western blot was performed with antibodies against Oct4 to monitor production of protein over the same time course. ESCs and endogenous Oct4 are shown as a positive control. β-Actin was used as a loading control. (H) Endogenous Pou5f1 expression was monitored in partially differentiated ESCs and the same cells treated with doxycycline for 12 h using qRT-PCR. Undifferentiated ESCs were used as a control. (I) ChIP was performed at the Pou5f1 enhancer in 11-day RA-differentiated ESCs and after 10 h of induction with doxycycline as in panel D; however, DMOG was also added after 4 h of doxycycline treatment. Cells were therefore exposed to doxycycline for 10 h and to DMOG for 6 h. Jmjd1c, H3K9me2, Spt16, and H3 antibodies were used.