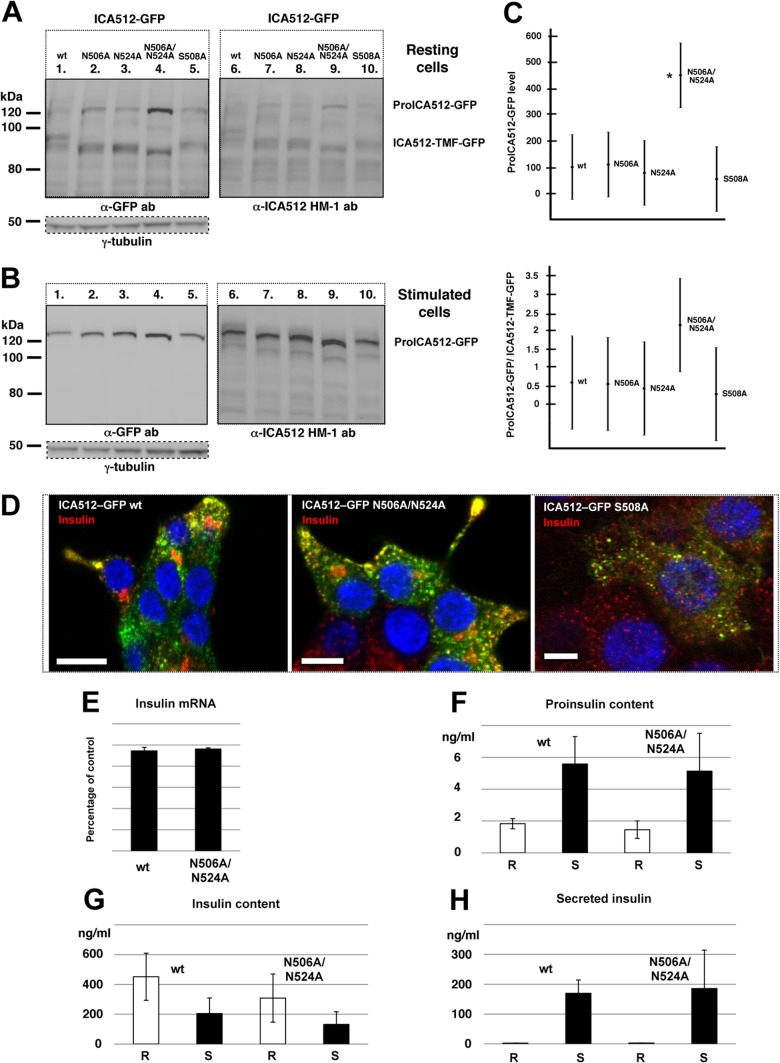
FIG 3.
Perturbing N-glycosylation or the ME ICA512 β2-strand does not prevent ICA512 targeting to SGs. (A and B) Immunoblotting for GFP or ICA512 in lysates of resting (A) or stimulated (B) INS-1 cells transfected with either wild-type (wt) ICA512-GFP or the N506A, N524A, N506A/N524A, or S508A mutant (n ≥ 3). For normalization, the same cell lysates were also immunoblotted for γ-tubulin. (C) Quantification by ANOVA of proICA512-GFP species (top) and of the ratios of the corresponding proICA512-GFP/ICA512-TMF-GFP species (bottom). The mean ± standard error of the mean (SEM) for each ICA512 species was normalized to that for γ-tubulin in the same lysates. *, P < 0.005 (n = 3). (D) Confocal microscopy images of HGHK-stimulated INS-1 cells transfected with ICA512-GFP or the corresponding N506A/N524A or S508A mutant (green) and immunostained for insulin (red) (n ≥ 3). Nuclei were labeled with DAPI (4′,6-diamidino-2-phenylindole) (blue). Bars = 5 μm. (E) Levels of insulin mRNA in ICA512-GFP wt and ICA512-GFP N506A/N524A sorted cells as measured by reverse transcription-PCR (RT-PCR) and normalized for β-actin mRNA levels (n = 3). Levels of proinsulin (F) and insulin (G) in ICA512-GFP wt and ICA512-GFP N506A/N524A sorted cells kept at rest (R) or stimulated (S) for 2 h with HGHK were measured by ELISA (n = 3). (H) Insulin secretion measured from ICA512-GFP wt and ICA512-GFP N506A/N524A sorted cells kept at rest (R) or stimulated (S) for 2 h with HGHK (n = 3).