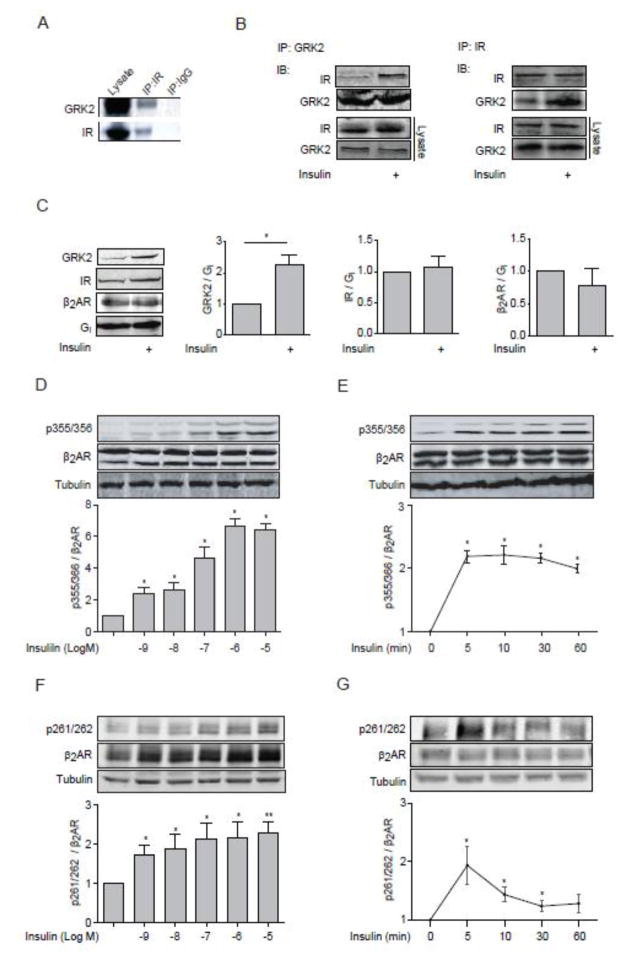
Figure 1. Insulin induces GRK2 membrane translocation and GRK-mediated phosphorylation of β2AR.
(A) Mouse heart lysates were subjected to immunoprecipitation with anti-IR antibody. (B) WT MEF cells were lysed for immunoprecipitation with either an anti-IR antibody or an anti-GRK2 antibody upon insulin stimulation (100 nM, 10 min). Immunoprecipitated proteins were detected by Western blot. Data represent at least three independent experiments. (C) H9c2 cardiac myoblasts overexpressed β2AR were stimulated with insulin (100 nM, 10 min). Membrane fractions were blotted for GRK2, IR, β2AR, and Gi. The protein levels on the plasma membrane were normalized to those of Gi. * p < 0.05 by student t-test relative to control group ( n = 3). (D–G) H9c2 cardiac myoblasts were infected with adenovirus expressing mouse β2AR and then stimulated with insulin for 10 min at indicated doses (D and F) or with 100 nM insulin for indicated times (E and G). The levels of phosphorylation of β2AR at serine 261/262 and 355/356 were detected by western blot, and normalized against total β2AR (n = 5). * p < 0.05 and ** p < 0.01 by one-way ANOVA in relative to control followed by Tukey’s test.