Figure 5.
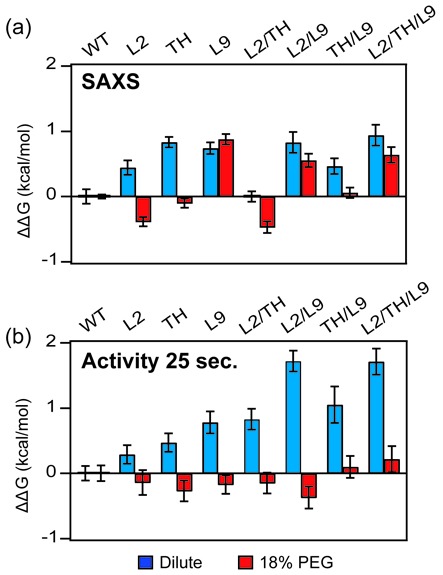
Crowding stabilizes the folded ribozymes. Relative folding free energies ΔΔG = ΔGmut – ΔGWT for (a) U→IC transition measured by SAXS and (b) the IC→N transition measured by activity (25 s). The folding free energies were evaluated at [Mg2+]1/2 for the WT ribozyme in each solution condition: 0.47 mM MgCl2 (SAXS, dilute), 0.28 mM (SAXS, 18% PEG), 1.56 mM (activity, dilute) and 1.34 mM MgCl2 (activity, 18% PEG). Error bars depict the standard deviation calculated from 10 000 resampling of fit residuals. The free energy obtained from activity assay is normalized to maximum activity of each RNA (Supplementary Figure S2). The [Mg2+]1/2 values of the WT and single mutant ribozymes reported here are lower than previously reported (10), owing to the 25 s quench used here, versus <20 s quench used previously. The longer reaction interval allows a portion of semi-native intermediates to refold, boosting the relative activity of the WT ribozyme and making the relative effects of multiple mutations more apparent (Supplementary Figure S7).
