Figure 3.
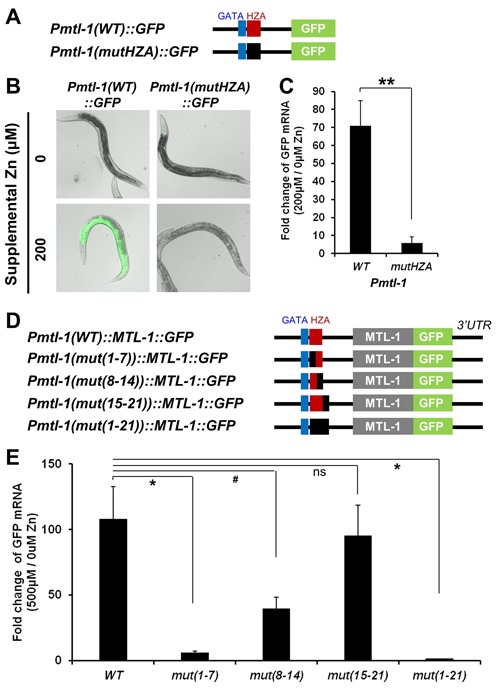
The HZA element is necessary for mtl-1 induction in response to high dietary zinc. (A and D) Diagrams (not to scale) of the mtl-1 promoter region extending 489 bp upstream of the translation start codon (black line) that was fused to the coding region for GFP (panel A) or had the coding region of GFP inserted at the position of the stop codon (panel D). The fragment includes the wild-type GATA element (blue box), and the wild-type HZA element (red box) or mutated HZA elements (black box) consisting of scrambled nucleotide sequence. The MTL-1 coding region (gray box) and the 3′UTR are shown in panel D. (B) Images show transgenic animals containing the indicated plasmids that were cultured with 0 or 200 μM supplemental zinc at L4/adult stage and imaged by fluorescence microscopy. GFP fluorescence signals (green) were captured with the identical settings and exposure times and overlaid with bright field images. (C) Transgenic animals containing reporter constructs shown in panel A were cultured with 0 or 200 μM supplemental zinc, and GFP mRNA levels were analyzed by qRT-PCR. The bars indicate the fold change of GFP mRNA levels as the ratio between the level at 200 and 0 μM supplemental zinc. A positive value indicates induction in high zinc. Values are the average ± SEM of three independent experiments. The mutated promoter was significantly different than the wild-type promoter (**P < 0.01). (D and E) Transgenic animals containing reporter constructs shown in panel D were cultured with 0 or 500 μM supplemental zinc, and GFP mRNA levels were analyzed by qRT-PCR. The bars indicate the fold change of GFP mRNA levels as the ratio between the level at 500 and 0 μM supplemental zinc. A positive value indicates induction in high zinc. Values are the average ± SEM of three independent experiments. The mutated promoters are compared to the wild-type promoter (*P < 0.05, #P = 0.06, ns P = 0.73). 500 μM supplemental zinc is a high concentration that causes toxicity with continuous culture; a short exposure to this high concentration of zinc was used in this experiment to maximize the response of mutant promoters and improve our ability to measure a small level of induction.
