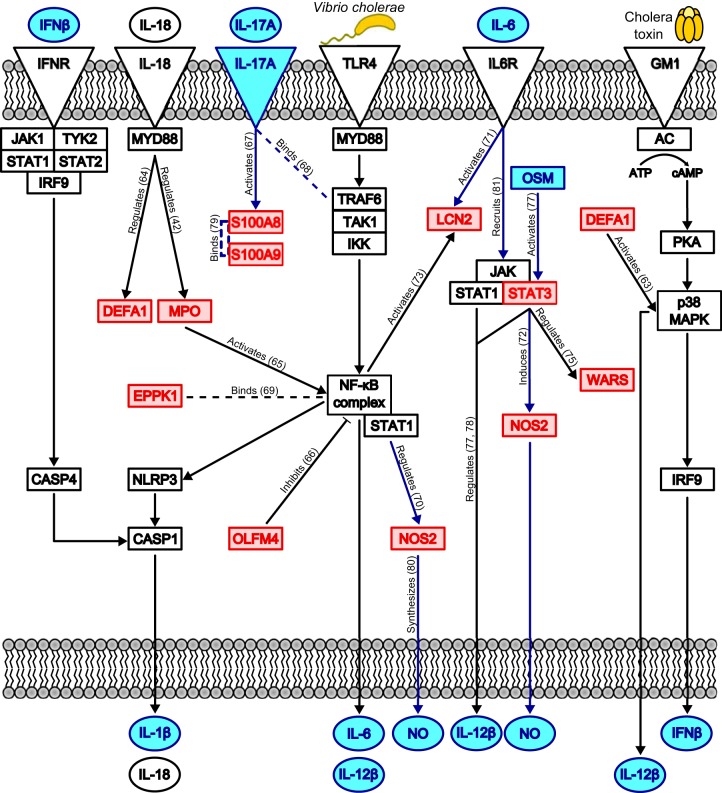
FIG 6.
Proposed human innate immune signaling pathways activated during V. cholerae O1 infection of the duodenum. We developed this model by combining our proteomic results and the results of the pathway analyses using Ingenuity's IPA software. Our model draws connections between differentially abundant proteins in our data set (red boxes), predicted upstream regulators (blue boxes and circles), enriched canonical pathways (blue lines), and proteins/pathways known to respond to V. cholerae and cholera toxin (CT) in vitro (black boxes, circles, and lines). Dashed lines represent binding interactions, whereas solid lines with arrowheads indicate activation or regulation of a downstream protein. Solid lines with blunt ends indicate repression of a downstream protein. Upon exposure to V. cholerae, TLR4 is stimulated, which leads to activation of NF-κB and the NLRP3 inflammasome; concurrently, CT stimulates G-protein-coupled receptors (GM1), which activate p38 MAPK. These inflammatory mediators drive production of the cytokines IL-1β, IL-18, IL-6, IL-12β, and IFN-β; NOS2 is also activated, leading to nitric oxide (NO) production. IL-6, IFN-β, and IL-18 function in autocrine loops to further amplify their own production, the inflammatory response via NF-κB and NLRP3, and cell death by apoptosis. IL-17A produced by activated T cells aids in regulating innate defense protein production, such as that of S100A8 and S100A9. Interactions found using Ingenuity's Knowledge Base are labeled with actions, and references are given in parentheses (42, 63–81). IFNR, IFN receptor.