FIG 1.
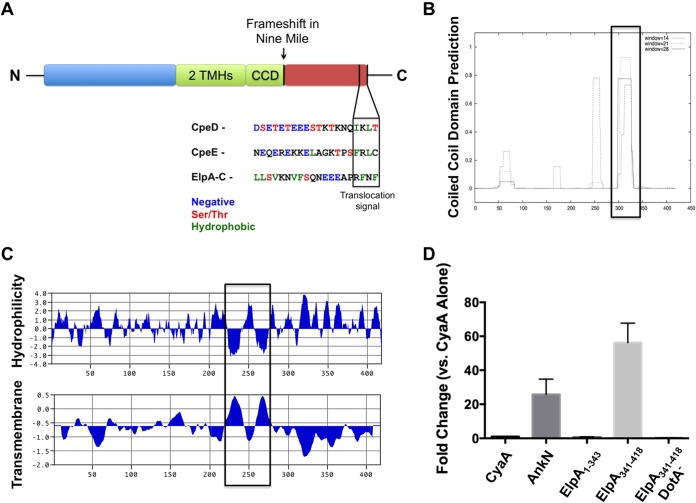
CbuD1884 encodes a Dot/Icm substrate with protein-protein interaction domains. (A) Schematic of the CbuD1884 protein (ElpA) containing two transmembrane helices (TMHs), a coiled-coil domain (CCD), and a putative C-terminal translocation signal. ElpA is full length in all C. burnetii isolates except the Nine Mile reference isolate, where a frameshift mutation (arrow) results in a pseudogene. The Dot/Icm substrates CpeD and CpeE are shown for C-terminal comparisons, and the putative Dot/Icm translocation signal is shown in the boxed region. The ElpA amino acid sequence was analyzed with COILS (B) and MacVector (C). ElpA contains two hydrophobic regions between residues 224 and 280, and these regions correspond to the presence of two TMHs. COILS analysis shows a CCD at residues 298 to 331. Boxes denote regions of interest. These results suggest the functional domains of ElpA reside in the N-terminal region (amino acids 1 to 331) of the protein. (D) In an established Dot/Icm translocation assay, L. pneumophila expressing the indicated proteins fused to CyaA was allowed to infect THP-1 cells for 30 min, and then cAMP levels were determined as a reporter for delivery to the cytosol. CyaA was used as a nonsecreted negative control and AnkN was a positive secreted control (5). Experiments were performed in triplicate, and error bars represent the standard deviations from the means. Elevated levels of cAMP indicative of secretion were observed for ElpA341–418 when it was expressed by wild-type L. pneumophila but not the DotA mutant (DotA−). These results show that only the C-terminal region of ElpA, not the N-terminal portion (ElpA1–343), contains the Dot/Icm T4SS translocation signal.