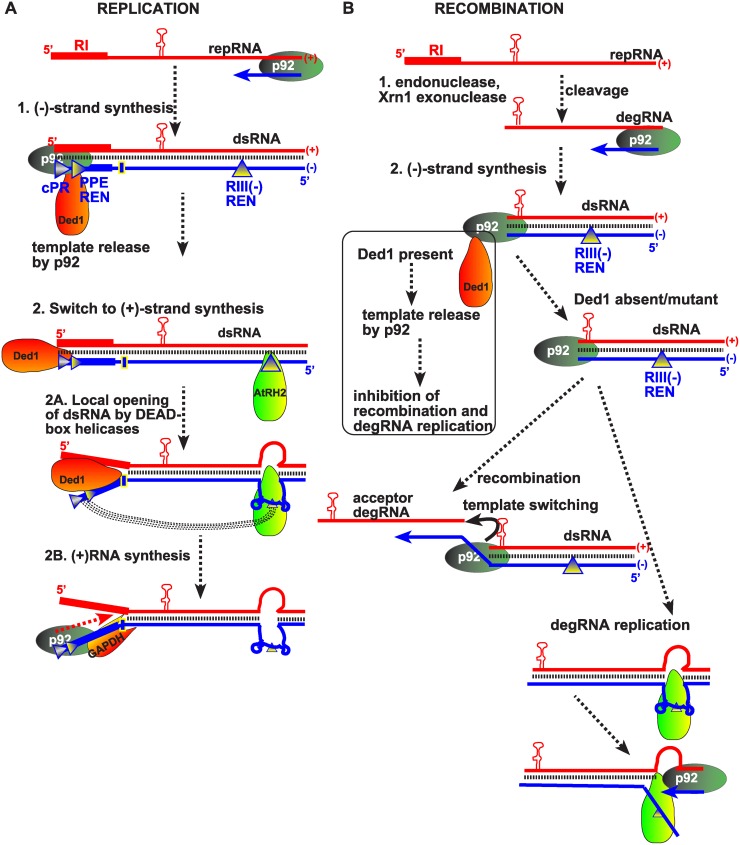
Fig 9. Models showing the functions of subverted cellular DEAD-box helicases in TBSV replication and viral RNA recombination.
(A) We propose that the DDX3-like Ded1/AtRH20 helicase facilitates the release of the pausing viral RdRp (shown as p92) at the end of the template, leading to switch from (-)-strand synthesis to (+)-strand synthesis. The previously defined additional functions of Ded1p and the eIF4AIII-like AtRH2 helicase is to open the dsRNA at the promoter region (cPR plus promoter proximal replication enhancer, PPE REN, indicated by rectangles) and the RIII(-)replication enhancer, as shown schematically [49,54,61]. Also shown is the long-range RNA-RNA interaction between the RIII(-) REN and the cPR region, which is proposed to help repeated use of the (-)RNA of the dsRNA as template for (+)RNA synthesis. The hairpin structure indicates the critical RII(+)-SL sequence required for the p33 replication protein-driven recruitment of the viral (+)RNA into replication. The RI(+) and the complementary RI(-) RNA sequences bound by Ded1p are shown with wide bars. (B) Our model suggests that Ded1/AtRH20 suppresses viral RNA recombination by facilitating the release of the pausing p92 at the end of degRNA recombinogenic template (created by cellular nucleases as shown). This activity of Ded1p results in suppression of RNA recombination (template-switching by p92), see the boxed portion of the model. Depletion of Ded1p or when Ded1p is mutated (ded1–199ts), p92 could stay on the degRNA template that likely promotes template-switching to a new (+)degRNA as shown as acceptor degRNA or to the 3’ end of the same (+)degRNA. On the other hand, in the absence of Ded1p, the eIF4AIII-like AtRH2 helicase could facilitate (-)RNA synthesis by opening up the dsRNA intermediate of degRNA that might lead to re-loading p92 RdRp to the 3’end of the (+)-strand for a new round of (-)degRNA synthesis. Similar activity by AtRH2 on degRNA might also facilitate template-switching by p92 RdRp for more efficient recombination. Altogether, these models take into account the co-ordinated actions of the two groups of subverted cellular helicases during replication and recombination.