Figure 5.
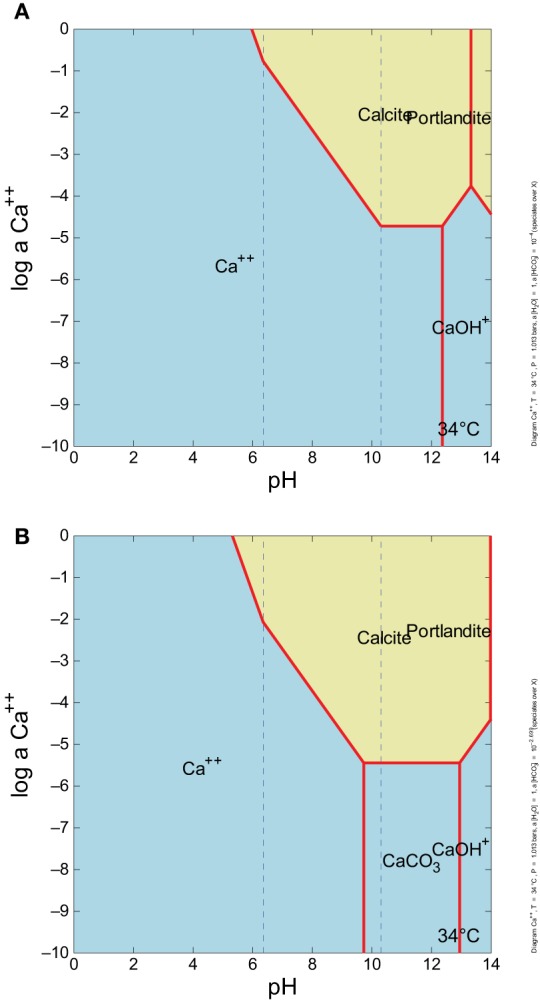
Ca+2 activity vs. pH, with (A) ~0.0001 m HCO−3 (which is equivalent to 1 ppm total C as DIC), and (B) ~0.002 m HCO−3, about what is in surface water. There is no CaCO3 aqueous complex field in the (A), but there is in (B). As CO2 increases, the aqueous CaCO3 complex field forms, and the stability field of Portlandite (CaOH2) shifts to the right.
