Abstract
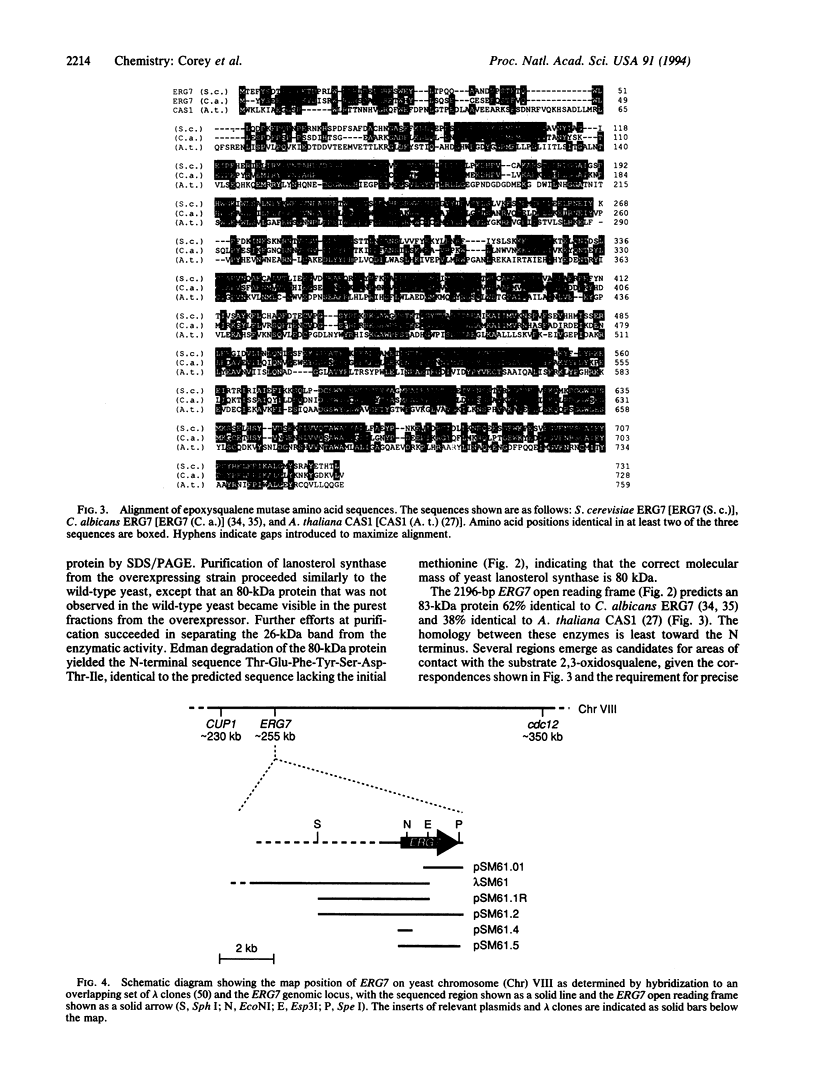
We report the cloning, characterization, and overexpression of Saccharomyces cerevisiae ERG7, which encodes lanosterol synthase [(S)-2,3-epoxysqualene mutase (cyclizing, lanosterol forming), EC 5.4.99.7], the enzyme responsible for the complex cyclization/rearrangement step in sterol biosynthesis. Oligonucleotide primers were designed corresponding to protein sequences conserved between Candida albicans ERG7 and the related Arabidopsis thaliana cycloartenol synthase [(S)-2,3-epoxysqualene mutase (cyclizing, cycloartenol forming), EC 5.4.99.8]. A PCR product was amplified from yeast genomic DNA using these primers and was used to probe yeast libraries by hybridization. Partial-length clones homologous to the two known epoxysqualene mutases were isolated, but a full-length sequence was found neither in cDNA nor genomic libraries, whether in phage or plasmids. Two overlapping clones were assembled to make a functional reconstruction of the gene, which contains a 2196-bp open reading frame capable of encoding an 83-kDa protein. The reconstruction complemented the erg7 mutation when driven from either its native promoter or the strong ADH1 promoter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. S., Muehlbacher M., Street I. P., Proffitt J., Poulter C. D. Isopentenyl diphosphate:dimethylallyl diphosphate isomerase. An improved purification of the enzyme and isolation of the gene from Saccharomyces cerevisiae. J Biol Chem. 1989 Nov 15;264(32):19169–19175. [PubMed] [Google Scholar]
- Anderson M. S., Yarger J. G., Burck C. L., Poulter C. D. Farnesyl diphosphate synthetase. Molecular cloning, sequence, and expression of an essential gene from Saccharomyces cerevisiae. J Biol Chem. 1989 Nov 15;264(32):19176–19184. [PubMed] [Google Scholar]
- Arthington B. A., Bennett L. G., Skatrud P. L., Guynn C. J., Barbuch R. J., Ulbright C. E., Bard M. Cloning, disruption and sequence of the gene encoding yeast C-5 sterol desaturase. Gene. 1991 Jun 15;102(1):39–44. doi: 10.1016/0378-1119(91)90535-j. [DOI] [PubMed] [Google Scholar]
- Arthington B. A., Hoskins J., Skatrud P. L., Bard M. Nucleotide sequence of the gene encoding yeast C-8 sterol isomerase. Gene. 1991 Oct 30;107(1):173–174. doi: 10.1016/0378-1119(91)90314-2. [DOI] [PubMed] [Google Scholar]
- Ashman W. H., Barbuch R. J., Ulbright C. E., Jarrett H. W., Bard M. Cloning and disruption of the yeast C-8 sterol isomerase gene. Lipids. 1991 Aug;26(8):628–632. doi: 10.1007/BF02536427. [DOI] [PubMed] [Google Scholar]
- Ballester R., Michaeli T., Ferguson K., Xu H. P., McCormick F., Wigler M. Genetic analysis of mammalian GAP expressed in yeast. Cell. 1989 Nov 17;59(4):681–686. doi: 10.1016/0092-8674(89)90014-7. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey E. J., Matsuda S. P., Bartel B. Isolation of an Arabidopsis thaliana gene encoding cycloartenol synthase by functional expression in a yeast mutant lacking lanosterol synthase by the use of a chromatographic screen. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11628–11632. doi: 10.1073/pnas.90.24.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory E. J., Russey W. E., Ortiz de Montellano P. R. 2,3-oxidosqualene, an intermediate in the biological synthesis of sterols from squalene. J Am Chem Soc. 1966 Oct 20;88(20):4750–4751. doi: 10.1021/ja00972a056. [DOI] [PubMed] [Google Scholar]
- Dean P. D., Ortiz de Montellano P. R., Bloch K., Corey E. J. A soluble 2,3-oxidosqualene sterol cyclase. J Biol Chem. 1967 Jun 25;242(12):3014–3015. [PubMed] [Google Scholar]
- Fegueur M., Richard L., Charles A. D., Karst F. Isolation and primary structure of the ERG9 gene of Saccharomyces cerevisiae encoding squalene synthetase. Curr Genet. 1991 Nov;20(5):365–372. doi: 10.1007/BF00317063. [DOI] [PubMed] [Google Scholar]
- Gaber R. F., Copple D. M., Kennedy B. K., Vidal M., Bard M. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol. 1989 Aug;9(8):3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Dayan J., Adlersberg M., Sprinson D. B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J Biol Chem. 1977 May 10;252(9):2846–2854. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Jandrositz A., Turnowsky F., Högenauer G. The gene encoding squalene epoxidase from Saccharomyces cerevisiae: cloning and characterization. Gene. 1991 Oct 30;107(1):155–160. doi: 10.1016/0378-1119(91)90310-8. [DOI] [PubMed] [Google Scholar]
- Jennings S. M., Tsay Y. H., Fisch T. M., Robinson G. W. Molecular cloning and characterization of the yeast gene for squalene synthetase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6038–6042. doi: 10.1073/pnas.88.14.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst F., Lacroute F. Ertosterol biosynthesis in Saccharomyces cerevisiae: mutants deficient in the early steps of the pathway. Mol Gen Genet. 1977 Sep 9;154(3):269–277. doi: 10.1007/BF00571282. [DOI] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Lai M. H., Kirsch D. R. Cloning and characterization of the 2,3-oxidosqualene cyclase-coding gene of Candida albicans. Gene. 1990 Mar 15;87(2):177–183. doi: 10.1016/0378-1119(90)90299-7. [DOI] [PubMed] [Google Scholar]
- Liu H., Krizek J., Bretscher A. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics. 1992 Nov;132(3):665–673. doi: 10.1093/genetics/132.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. T., Casey W. M., Parks L. W. Structural discrimination in the sparking function of sterols in the yeast Saccharomyces cerevisiae. J Bacteriol. 1989 Nov;171(11):6169–6173. doi: 10.1128/jb.171.11.6169-6173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. T., Parks L. W. Cloning, sequencing, and disruption of the gene encoding sterol C-14 reductase in Saccharomyces cerevisiae. DNA Cell Biol. 1992 Nov;11(9):685–692. doi: 10.1089/dna.1992.11.685. [DOI] [PubMed] [Google Scholar]
- Oulmouden A., Karst F. Isolation of the ERG12 gene of Saccharomyces cerevisiae encoding mevalonate kinase. Gene. 1990 Apr 16;88(2):253–257. doi: 10.1016/0378-1119(90)90039-t. [DOI] [PubMed] [Google Scholar]
- Oulmouden A., Karst F. Nucleotide sequence of the ERG12 gene of Saccharomyces cerevisiae encoding mevalonate kinase. Curr Genet. 1991 Jan;19(1):9–14. doi: 10.1007/BF00362081. [DOI] [PubMed] [Google Scholar]
- Parks L. W., Rodriguez R. J., Low C. An essential fungal growth factor derived from ergosterol: a new end product of sterol biosynthesis in fungi? Lipids. 1986 Jan;21(1):89–91. doi: 10.1007/BF02534308. [DOI] [PubMed] [Google Scholar]
- Ramgopal M., Bloch K. Sterol synergism in yeast. Proc Natl Acad Sci U S A. 1983 Feb;80(3):712–715. doi: 10.1073/pnas.80.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riles L., Dutchik J. E., Baktha A., McCauley B. K., Thayer E. C., Leckie M. P., Braden V. V., Depke J. E., Olson M. V. Physical maps of the six smallest chromosomes of Saccharomyces cerevisiae at a resolution of 2.6 kilobase pairs. Genetics. 1993 May;134(1):81–150. doi: 10.1093/genetics/134.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., Taylor F. R., Parks L. W. A requirement for ergosterol to permit growth of yeast sterol auxotrophs on cholestanol. Biochem Biophys Res Commun. 1982 May 31;106(2):435–441. doi: 10.1016/0006-291x(82)91129-9. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Ryder N. S. Inhibition of squalene epoxidase and sterol side-chain methylation by allylamines. Biochem Soc Trans. 1990 Feb;18(1):45–46. doi: 10.1042/bst0180045. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser D., Nonet M., Young R. A. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1192–1196. doi: 10.1073/pnas.84.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay Y. H., Robinson G. W. Cloning and characterization of ERG8, an essential gene of Saccharomyces cerevisiae that encodes phosphomevalonate kinase. Mol Cell Biol. 1991 Feb;11(2):620–631. doi: 10.1128/mcb.11.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]



