Abstract
Background: To gain full benefit from disease-modifying therapies such as interferon β-1b, patients with multiple sclerosis (MS) need to adhere to treatment in the long term. Treatment adherence requires high patient satisfaction with treatment and care. Objectives: Our aim was to evaluate the satisfaction of patients with MS receiving interferon β-1b Extavia with the patient care program Extracare. Efficacy and safety of treatment were evaluated as secondary objectives. Methods: In this prospective, noninterventional 1-year study, data on the satisfaction of 174 patients with MS with Extracare were obtained by questionnaires. Disability and symptom severity as well as patients’ reported activity limitations, quality of life, and fatigue were recorded. Results: We observed high levels of patients’ satisfaction with MS nurses, telephonic care, and information provided by Extracare (values ≤ 1.53 on a Likert scale ranging from 1 [very good] to 6 [insufficient]). Patient reported quality of life (Patient Reported Indices for MS QoL) improved from 11.82 ± 11.36 at baseline to 9.74 ± 10.94 at the end of the study (p = .02), whereas clinical parameters of disease progression remained unchanged. Rate of adverse events was as expected. Conclusions: This study provides the basis for further improvements of care programs to increase treatment adherence of patients with MS.
Keywords: beta 1 interferon, medication adherence, multiple sclerosis, patient satisfaction
Multiple sclerosis (MS), a chronic demyelinating and neurodegenerative disorder of the central nervous system (Bennett & Stuve, 2009), results in various locomotor, sensory, and cognitive impairments (Keegan & Noseworthy, 2002). The course of the disease is still highly variable and unpredictable. However, since 1993, disease-modifying therapies (DMTs) have been shown to reduce relapse rates, to improve disease markers in magnetic resonance imaging studies, and to slow disease progression.
Among DMTs for MS, interferon β-1a (IFN β-1a), IFN β-1b such as Extavia/Betaferon, and glatiramer acetate have dominated MS practice and are recommended in current guidelines. In relapsing-remitting MS (RRMS), the most common MS subtype, both interferons are comparably effective, with a slight dominance of IFN β-1b over IFN β-1a (Barbero et al., 2006). Efficacy of IFN β-1b was tested in several trials. In a phase 3 trial on 372 patients with RRMS, IFN β-1b reduced the annual relapse rate by 34% and increased the rate of relapse-free patients to 31% when compared with placebo (16%) over 2 years (The IFNB Multiple Sclerosis Study Group, 1993).
However, to gain the full potential benefit from treatment, early and continuous use of DMTs is essential. In patients with clinically isolated syndromes suggestive of MS, IFN β-1b treatment delayed the conversion to clinically definite MS (Kappos et al., 2006). In patients with RRMS, early onset of DMT and high level of adherence have been associated with better long-term outcomes (Carroll, 2009; Goodin & Bates, 2009), better quality of life (QoL), and fewer neuropsychological issues (Devonshire et al., 2011). Accordingly, delayed treatment or low level of adherence has been shown to provide less benefit than continuous treatment (Carroll, 2009).
Three factors frequently constrain adherence to MS therapy. First, patients who did not experience relapses or symptoms for long periods find it difficult to accept the necessity of chronic treatment (Costello, Kennedy, & Scanzillo, 2008). Conversely, patients might question the efficacy of treatment if an aggravation of the disease occurs, although they have adhered to therapy (Fraser, Morgante, Hadjimichael, & Vollmer, 2004). Second, all currently used DMTs require regular parenteral administration, mostly by self-injection, with frequencies of administration varying from daily to weekly. Injection anxiety is a burden for many patients that can obviously lead to treatment discontinuation (Portaccio & Amato, 2009). Third, occurrence of adverse events (AEs) such as injection-site reactions and flu-like symptoms or accompanying symptoms of MS such as depression, fatigue, or cognitive impairment may additionally reduce the likelihood that patients adhere to therapy (Tremlett & Oger, 2003).
As a result, levels of adherence to DMTs are usually lower than optimal. A survey among patients with MS receiving DMT showed that about 40% of patients were nonadherent (Treadaway et al., 2009), and rates of discontinuation in clinical studies have been reported as up to 46% after a 4-year follow-up (Portaccio, Zipoli, Siracusa, Sorbi, & Amato, 2008). Patient care programs have therefore been established to promote the treatment adherence of patients with MS. Beside other patient programs, the patient care program Extracare has been set up to support the adherence to IFN β-1b Extavia. Patient care programs have recently been introduced in MS for other pharmaceuticals (e.g., MS-Begleiter for alemtuzumab) as well as in different indications, for example, patient care programs FocalCare (ranibizumab) and VisusVital (aflibercept) in ophthalmology.
MS nurses play the central role within patient care programs such as Extracare by delivering training on injection techniques, by helping with the management of symptoms and side effects, and by encouraging patients to adhere to their medication (Ross, 2008). In the Extracare program, patient education by nurses is further supplemented by telephone counseling and information material, helping patients both to better understand their condition and to have a more realistic expectation of treatment outcome. Moreover, modern drug delivery devices such as self-injectors can additionally improve adherence (Lee, Balu, Cobden, Joshi, & Pashos, 2006).
A known prerequisite for optimal therapy adherence is a high level of patients’ satisfaction with treatment, healthcare services, and information provided (Pascoe, 1983). The aim of this study was therefore to evaluate the satisfaction of patients with MS receiving IFN β-1b Extavia with the different components of the patient care program Extracare. This report provides a status quo analysis of the patients’ acceptance of Extracare, thereby identifying potential improvements of future programs to enhance treatment adherence.
Methods
Participants and Procedures
This noninterventional study was performed between December 2009 and September 2011 in 77 neurological practices in Germany. The study cohort included 174 patients diagnosed with clinically definite MS (Polman et al., 2005). Patients were eligible if they were older than 18 years and if their physician had already decided to prescribe Extavia. Further inclusion criteria were that the patient had registered with the Extracare program offered by Novartis, Germany, and had given written informed consent (Novartis Pharma GmbH, 2012).
The Extracare program provides patients with access to specially trained MS nurses who offer practical education on injection techniques and management of side effects. Enrolled patients further received telephone consultations by the Extracare service center that also organized visits by nurses to patients’ homes if necessary. In addition, Web- and print-based media provided information about the medication, including storage and transportation, possible side effects, and importance of adherence. If desired, patients received a starter bag for carrying the medication, a written guide, and a DVD on how to perform the injections.
This study was designed as a prospective, longitudinal study to assess the patients’ acceptance of the Extracare program as the primary objective and to evaluate the safety and efficacy of Extavia treatment as the secondary objective. Medical consultations were conducted at baseline and every 3 months, and data on both clinical parameters and patients’ satisfaction were recorded over a period of 12 months of Extavia therapy (250 µg [1 ml] injected subcutaneously every other day; Novartis Pharma GmbH, 2009).
Questionnaire
Patients’ acceptance of and satisfaction with the Extracare program were assessed by questionnaires at 3, 6, 9, and 12 months; physicians were interviewed by questionnaires after 6 months. Patients and physicians rated their satisfaction with different aspects of MS nursing care and telephonic care on 6-point Likert scales ranging from 1 (very satisfied) to 6 (very dissatisfied). In addition, patients rated their satisfaction with both injection training provided by MS nurses and frequency of visits. Patients were furthermore asked to rank different sources of information according to their perceived value on a 3-point Likert scale (high, medium, and low) with “do not know” as an additional choice. To assess the overall satisfaction of patients with the Extracare program, patients were asked to rank the perceived usefulness of the Extracare program on a 3-point Likert scale (useful, little useful, and useless). Patients were finally asked about their intention to participate in the Extracare program in the future and if they would recommend the program to other patients.
Clinical Data
Clinical data on disease progression and baseline characteristics such as concomitant diseases and treatment were collected by the physician at baseline visit and at visits after 3, 6, 9, and 12 months. Disability and symptom severity were scored by the Kurtzke’s Expanded Disability Status Scale (EDSS; Meyer-Moock, Feng, Dippel, & Kohlmann, 2013) and the Clinical Global Impression (CGI; Busner & Targum, 2007), respectively. The EDSS is a validated and widely used method for measuring disability and disability progression over time in MS, whereby the score ranges from 0 (normal neurological findings) up to 10 (death as result of MS disease). The scales are based on measures of impairment in eight functional systems: pyramidal, cerebellar, brainstem, sensory, bowel and bladder function, visual function, cerebral (or mental) functions, and ambulation index.
The CGI scale complements the EDSS analysis by focusing on assessing patients’ mental health status and is divided into two subscales. The CGI-Severity scale evaluates the impression of the severity of illness in seven units ranging from 0 = “not at all ill” up to 7 = “among the most extremely ill patients.” The second CGI subscale evaluates the global improvement compared with the start of study. The scales range from “very much worse” up to “very much improved.” The EDSS and CGI were recorded under typical patient care conditions in normal clinical practice.
To capture the overall impact of MS from the patient’s perspective, the Patient Reported Indices for MS (PRIMUS) scales (Doward, McKenna, Meads, Twiss, & Eckert, 2009) for activity limitations and QoL as well as the Modified Fatigue Impact Scale (Fisk et al., 1994) were assessed by patient interviews. The PRIMUS QoL score ranges from 0 to 22 and is defined by assessing the patient’s needs in form of simple statements supplemented with different response options. A high score depicts a worse QoL. The PRIMUS activities score ranges from 0 to 30 and contains 15 items. This score reflects the patient’s evaluation of MS affecting their day-to-day lives. High PRIMUS activity scores indicative greater activity limitations.
Treatment discontinuation was determined by patients’ self-reports or by the number of missed appointments, and reasons for premature discontinuation of treatment were documented by the physician by choosing appropriate statements from given options. The AEs were registered throughout the study period. Documentation of AEs and assessment of tolerability were performed by the physician.
Statistics
The statistical analysis followed a standardized operating procedure of the Clinical Research Organization SIMW GmbH and included all case report forms received, irrespective of any possible inconsistencies, incompleteness, or implausibilities (intent-to-treat collective). Unadjusted data were analyzed on an intent-to-treat basis by mainly descriptive statistical methods. Interval scaled parameters are presented as mean value ± standard deviation (SD), and ordinal variables, that is, frequency of responses, were expressed as frequency relative to the number of valid answers. Differences between pretreatment and posttreatment measures of clinical scores were tested for significance by matched-pairs tests. The statistical analyses were performed with Statistica v. 8.0.
Results
Baseline Characteristics
Baseline characteristics are summarized in Table 1. In brief, mean age was 40.22 ± 12.06 years, and most subjects were women (74.71%). On average, participants were diagnosed with MS 58.07 ± 70.30 months before enrollment and had mostly RRMS (71.84%). Approximately half of the participants (47.13%) had already been treated with immunomodulatory medication before adjustment to Extavia. Among the patients who had been changed from other medication, for 20 of the 169 patients (11.83%), dissatisfaction with handling or support and poor treatment adherence were reported as reasons for change. Cost considerations were reported for 38 patients (22.49%). Fifty-seven (32.76%) patients had previous and concomitant diseases, especially hypertension (7.47%) and depression (6.32%). Among concomitant medications and treatments, the mostly used medications were ibuprofen (9.77%) and levothyroxine natrium (6.90%).
TABLE 1.
Baseline Characteristics of Patients Enrolled in the Study
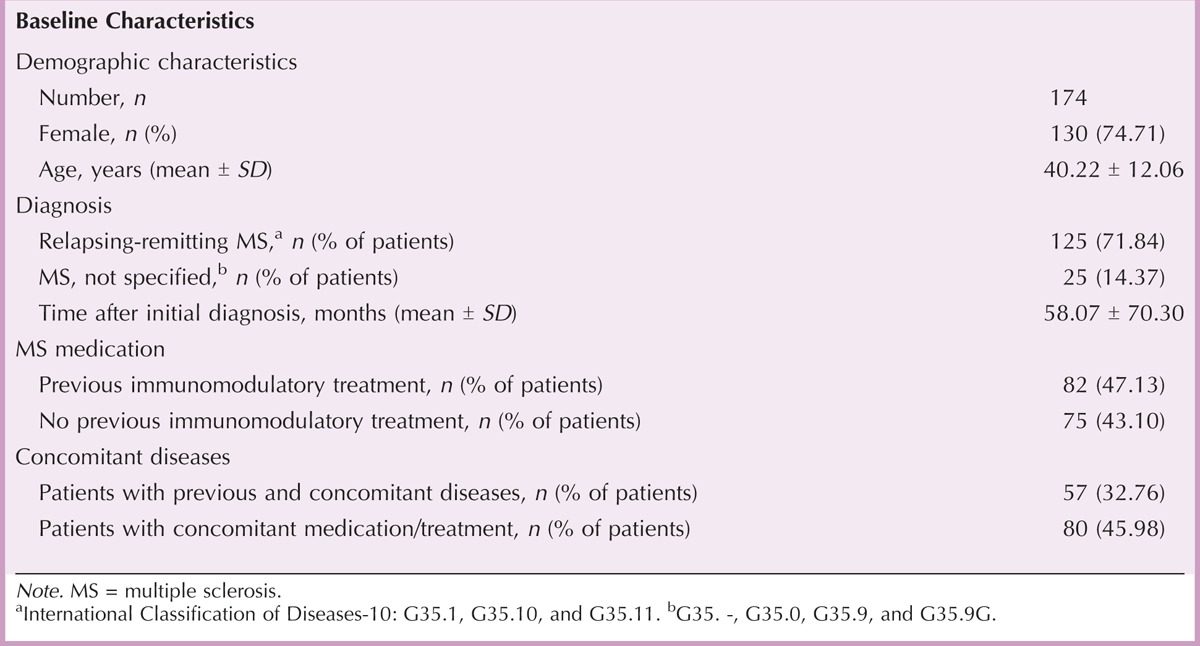
Satisfaction With MS Nurses and Injection Training
Of 113 physicians, 72 (63.72%) had an MS nurse in their medical department, whereas 41 (36.28%) cooperated with external partners. To evaluate the satisfaction with MS nurses, physicians and patients were asked to grade different aspects of nursing care on a 6-point Likert scale after 6 and 3 months, respectively. Physicians and patients reported a high satisfactory level with the professional and personal competence and the reliability of MS nurses. Patients considered both the explanation of medication and application as well as the injection training provided by MS nurses to be “very good” (Table 2), and 68 of the 105 physicians (64.76%) estimated the injection training as “very important.” Injection training might therefore have helped to promote the usage of autoinjectors, which increased from 45.22% (52 of 115 patients) at the beginning of the study to 85.34% (99 of 116 patients) at month 3 of the study.
TABLE 2.
Satisfaction of Physicians and Patients With MS Nursing Care on a Likert Scale Ranging from 1 (Very Good) to 6 (Insufficient)
After 12 months, overall patient satisfaction with MS nurses was 1.44 ± 1.01, and most patients (53 of 80, 66.25%) rated the frequency of visits by MS nurses as “just right.” Only 4 of 80 patients (5.00%) would have expected a higher frequency, and no patient considered the frequency to be too often.
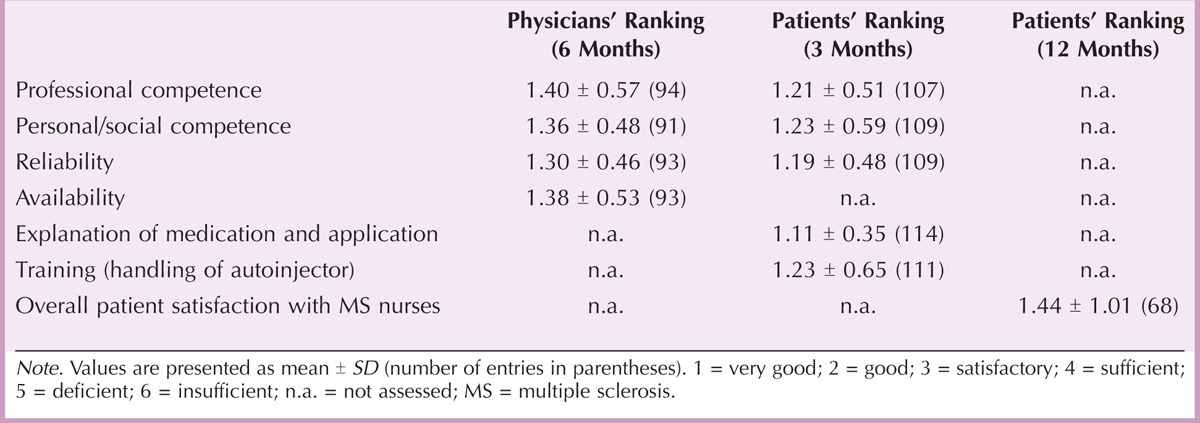
Satisfaction With Telephonic Care
After 6 months, physicians and patients indicated high satisfaction with different aspects of telephonic care provided by the Extracare service center. Among others, these aspects included availability, professional competence, kindness, and reliability (Table 3). At the end of the study, overall patient satisfaction with telephonic care was 1.43 ± 1.06 on a 6-point Likert scale. Forty-five of the 82 patients (54.88%) rated the frequency of calls received from the service center as “just right,” whereas 3 of the 82 patients (3.66%) would have expected a higher frequency. Five (0.06%) patients considered the frequency to be too often.
TABLE 3.
Satisfaction of Physicians and Patients With Telephonic Care on a Likert Scale Ranging from 1 (Very Good) to 6 (Insufficient)
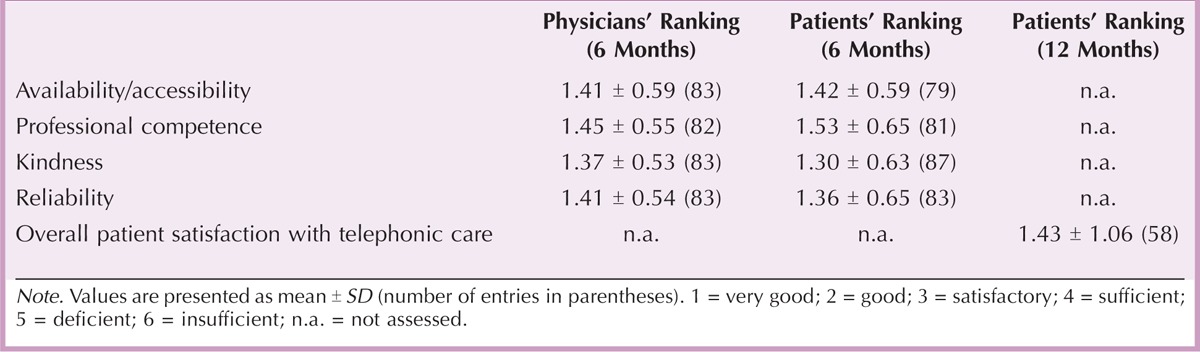
Satisfaction With Information Provided
At the end of the study, patients were further asked to rank the value of different sources of information. These sources were composed of the attending physician, the MS nurse, the service center, Internet, and print media as well as self-help groups and local activities for patients with MS. Table 4 shows that patients judged both physicians (92.68%) and MS nurses (62.20%) to be highly valuable sources of information, followed by Internet (41.33%) and print media (41.25%). However, less than one third of the patients (28.21%) considered the service center to be a highly valuable source of information.
TABLE 4.
Ranking of Different Information Sources by Patients After 12 Months
Overall Satisfaction With Extracare
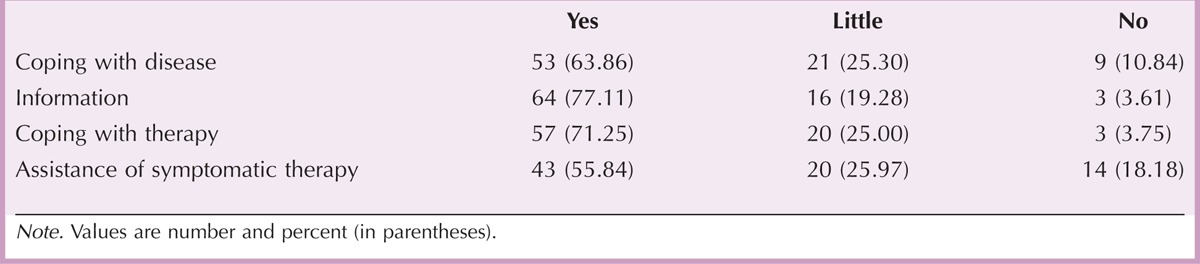
In a final assessment, patients were asked to judge the overall usefulness of Extracare (Table 5). In all categories provided (coping with disease or therapy, receiving MS-related information, assistance of symptomatic therapy), most patients considered the Extracare program as useful. However, almost half of the patients (34 of 77 patients, 44.16%) assigned little or no value to the assistance during symptomatic therapy, and 30 of 83 patients (36.14%) considered the support for coping with the disease to be only little useful or insufficient (Table 5). Finally, most patients intended to utilize certain elements of the program in the future (MS nurses [60.71%], information material [65.85%], service center [58.02%], Web-based information [41.03%]). Sixty-seven of 85 patients (78.82%) would recommend the Extracare program to other patients.
TABLE 5.
Patients’ Ranking of the Usefulness of the Extracare Program for Disease-Related Aspects
Disease Progression
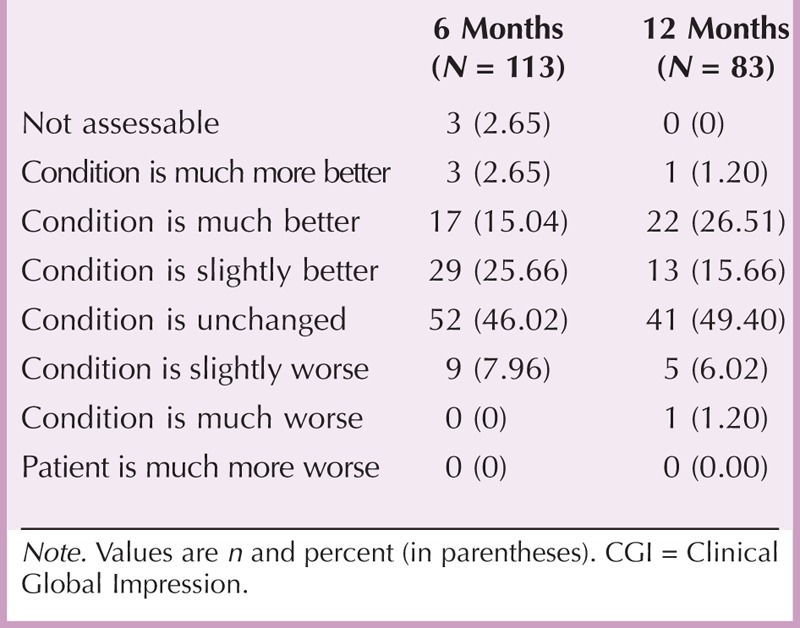
A secondary objective of the study was to investigate the efficacy and safety of Extavia treatment. Clinical parameters related to disease progression slightly improved during the study (Table 6). Mean sum scores of EDSS, PRIMUS activity, and Modified Fatigue Impact Scale decreased over 12 months, but differences between prestudy and poststudy values did not reach statistical significance. Accordingly, the percentage of patients who experienced at least slight improvements did not change after 6 (43.36%) and 12 (43.37%) months, as determined by CGI changes (Table 7). Condition of almost half of the patients was unchanged after 6 (46.02%) and 12 (49.40%) months. However, PRIMUS QoL sum scores significantly decreased from 11.82 ± 11.36 at baseline to 9.74 ± 10.94 at the end of the study (p = .02; Table 6), which indicates an improvement in QoL.
TABLE 6.
Assessment of Functional Disability (EDSS), Activity Limitations (PRIMUS Activity), Quality of Life (PRIMUS QoL), and Fatigue (mFIS)
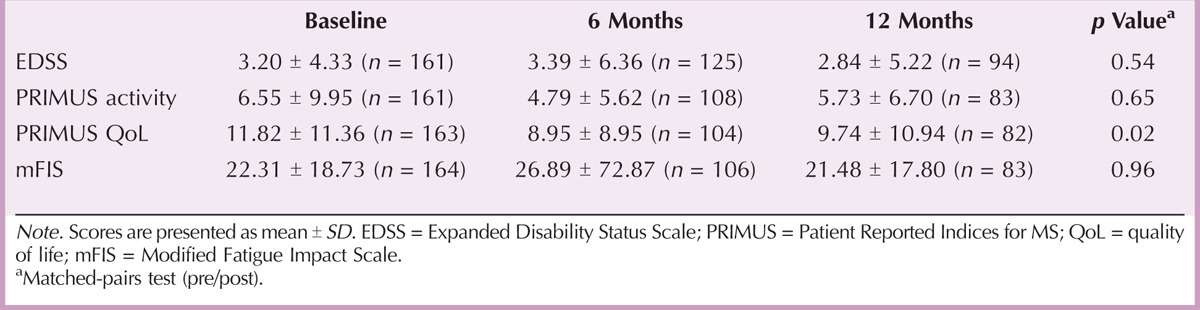
TABLE 7.
Changes in Symptom Severity (CGI) After 6 and 12 Months
AEs
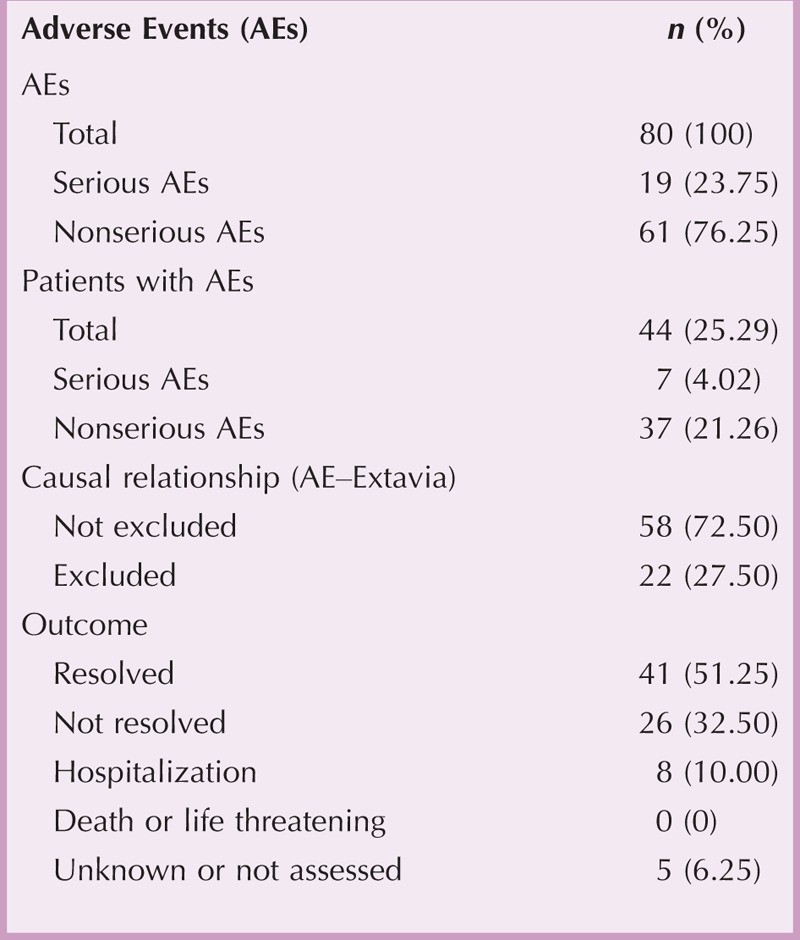
Forty-four of 174 patients (25.29%) reported 80 AEs. Half of AEs (51.25%) resolved during continued therapy, and eight AEs required hospitalization. There were no life-threatening AEs, and no patient died (Table 8). In 6 of 19 serious AEs (SAEs), the nervous system was affected. Other frequently observed SAEs were related to skin and subcutaneous disorders (three SAEs) and general disorders and administration site conditions (three SAEs). Non-SAEs mostly involved MS relapses (five AEs), injection-site indurations (five AEs), and flu-like symptoms (four AEs). In 58 AEs (72.50%), a causal relationship between AE and treatment with Extavia was at least “possible” or “not assessable” (Table 8).
TABLE 8.
Adverse Events During the Study Period
Therapy Adherence
Seventy-four of 174 patients (42.53%) prematurely terminated the study. Of these 74 patients, 42 stated reasons for discontinuation at regular visits. Among others, AEs (14), no satisfying efficacy (10), and poor compliance (9) were the most frequently stated reasons. However, most patients who completed the study indicated that they had regularly taken Extavia (79 of 87 patients, 90.80%). Likewise, most of these patients showed their intention for the future continuation of treatment with Extavia (87 of 99 patients, 87.88%).
Discussion
In this study, we observed a high level of patients’ satisfaction with the Extracare patient support program, as assessed by questionnaires. In particular, patients were very satisfied with MS nurses and telephonic care and judged both physicians and MS nurses to be highly valuable sources of information. Overall, patients ranked the Extracare program as useful in different disease-related aspects, and most patients would recommend the Extracare program to other patients. Accordingly, health-related QoL significantly improved during the study period. However, clinical parameters of disease progression remained unchanged; this might be because of the short study duration. This result is in accordance with the data of Rice et al. who showed that treatment for patients with RRMS using IFN β-1b improved QoL, especially in patients with an EDSS < 3.0 (Rice et al., 1999). The rate of AEs in our study was as expected. A limitation of this study might be the fact that, although most patients considered the Extracare program as useful, more than third of all patients rank the support for coping with MS to be only minor useful or even insufficient, and almost half of the patients granted little or no value of it in assistance during symptomatic therapy. This is comprehensible as MS is still a challenging disease for patients, and patient-orientated programs can only support the process to a certain degree. In the future, patient support programs therefore need a more patient-tailored approach to provide even better support.
Assessing to what extent patients with MS are satisfied with treatment, health services, and information provided is clinically relevant, because satisfied patients are more likely to adhere to therapy (Pascoe, 1983). Patients with MS who adhere closely to DMTs have the best prospects for decreasing the risk for relapses and for preserving functional and cognitive abilities (Devonshire et al., 2011). In the clinical setting, however, discontinuation rates of patients with MS of up to 46% have been reported (Lugaresi, 2009), and discontinuation is mostly observed within the first 2 years of treatment (Costello et al., 2008). Generally, discontinuation rates are lower in clinical trials than in clinical practice, as most trial participants are highly motivated.
Patient education programs are therefore becoming ever more important in the management of MS. With Extracare, we try to provide patients with MS with various types of assistance to enhance patients’ satisfaction with treatment. First of all, MS nurses are in an ideal position to provide supportive interventions such as injection training. In our study, patients ranked injection training by MS nurses as “very good,” and most physicians estimated the injection training as “very important.” The injection training is most likely responsible for the considerable increase of study participants who used autoinjectors. However, injection anxiety is a long-term barrier to adherence of individual patients even after several months of adaptation to sustained DMT and obviously requires psychological intervention (Turner, Williams, Sloan, & Haselkorn, 2009). To improve treatment adherence of patients with MS, it might be meaningful to provide MS nurses with an appropriate psychological education to help patients to overcome injection anxiety.
In our study, most patients judged the MS nurse to be a highly valuable source of information. The fact that most patients who stayed in the Extracare program in the long term reported both full compliance with Extavia and intention to continue treatment suggests that these patients are equipped with enough knowledge to fully understand the necessity of treatment adherence. However, some patients might require continuous health education via multiple channels that enhance their receptivity to new information. Because patients expressed only moderate levels of satisfaction with print or electronic media and telephone counseling, we conclude that these sources of information are in need for further improvement.
By its nature, nonadherence is difficult to measure empirically. An indirect measure such as patient self-reports might be biased by the patients’ tendency to overestimate or underestimate adherence. In our study, the dropout rate between baseline and final examination was as high as 42.53%, whereby it is unclear whether dropout patients only left the program or discontinued IFN β-1b therapy at all. Importantly, most patients who completed the study indicated that they had regularly taken Extavia and, furthermore, intended to continue treatment, which indicates the potential benefit patients receive from care programs. Among the patients who prematurely terminated our study, however, most patients mentioned AEs, no satisfying efficacy, or poor compliance as reasons for discontinuation. Likewise, empirical studies on DMT adherence identified side effects such as flu-like symptoms (Plosker, 2011) and injection-site reactions (Mohr et al., 1996) as well as perceived lack of treatment efficacy as the most frequently mentioned reasons for discontinuation (Kern, Reichmann, & Ziemssen, 2008). In our study, a considerable number of patients further stated “poor compliance” as reason for the complete discontinuation of treatment. Because about 50% of people with MS experience cognitive impairment to some degree (Potagas et al., 2008) and the most common deficit is memory loss (Mattioli et al., 2010), one reason for missing injections might be simply forgetting to take those (Treadaway et al., 2009). In addition, patients might also forget to alternate the injection site, which might increase the risk for injection-site reactions.
Despite the high levels of patients’ satisfaction, perceived lack of efficacy and AEs remain the most frequently stated reasons for nonadherence. Additional factors such as influences from the social environment may additionally affect adherence. In future patient support programs, we therefore plan to introduce further improvements: To provide a more individual support, MS nurses will be trained to provide specialized coaching for patients with MS at high risk for nonadherence, and furthermore, MS nurses with foreign language skills will be hired. Furthermore, the frequency of calls from the service center will be adapted to the individual requirements of patients. Finally, autoinjectors will be technically improved to simplify the usability and to minimize the risk for injection-site reactions.
In conclusion, we showed that patients with MS participating in the Extracare program were considerably satisfied with quality and usefulness of this program, which might essentially contribute to adherence to MS therapy. At the end of the study, health-related QoL was significantly improved, and treatment with Extavia was confirmed to be safe. The assessment of patients’ satisfaction therefore provides a valuable basis for further improvements to enhance therapy adherence of patients with MS in future care programs.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharma GmbH. We thank Dr. Stefan Lang for his medical editorial assistance with this manuscript.
Footnotes
This study was funded by Novartis Pharma GmbH.
The authors declare no conflicts of interest.
REFERENCES
- Barbero P., Bergui M., Versino E., Ricci A., Zhong J. J., Ferrero B., Durelli L. (2006). Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis (INCOMIN Trial) II: Analysis of MRI responses to treatment and correlation with NAb. Multiple Sclerosis, 12, 72– 76. [DOI] [PubMed] [Google Scholar]
- Bennett J. L., Stuve O. (2009). Update on inflammation, neurodegeneration, and immunoregulation in multiple sclerosis: Therapeutic implications. Clinical Neuropharmacology, 32, 121– 132. [DOI] [PubMed] [Google Scholar]
- Busner J., Targum S. T. (2007). The Clinical Global Impression Scale: Applying a research tool in clinical practice. Psychiatry, 4, 28– 37. [PMC free article] [PubMed] [Google Scholar]
- Carroll W. M. (2009). Clinical trials of multiple sclerosis therapies: Improvements to demonstrate long-term patient benefit. Multiple Sclerosis, 15, 951– 958. [DOI] [PubMed] [Google Scholar]
- Costello K., Kennedy P., Scanzillo J. ( 2008). Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape Journal of Medicine, 10, 225. [PMC free article] [PubMed] [Google Scholar]
- Devonshire V., Lapierre Y., Macdonell R., Ramo-Tello C., Patti F., Fontoura P., Kieseier B. C. (2011). The Global Adherence Project (GAP): A multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. European Journal of Neurology. 18, 69– 77. [DOI] [PubMed] [Google Scholar]
- Doward L. C., McKenna S. P., Meads D. M., Twiss J., Eckert B. J. (2009). The development of patient-reported outcome indices for multiple sclerosis (PRIMUS). Multiple Sclerosis, 15, 1092– 1102. [DOI] [PubMed] [Google Scholar]
- Fisk J. D., Ritvo P. G., Ross L., Haase D. A., Marrie T. J., Schlech W. F. (1994). Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clinical Infectious Diseases, 18 (Suppl 1), S79– S83. [DOI] [PubMed] [Google Scholar]
- Fraser C., Morgante L., Hadjimichael O., Vollmer T. (2004). A prospective study of adherence to glatiramer acetate in individuals with multiple sclerosis. Journal of Neuroscience Nursing, 36, 120– 129. [DOI] [PubMed] [Google Scholar]
- Goodin D. S., Bates D. (2009). Treatment of early multiple sclerosis: The value of treatment initiation after a first clinical episode. Multiple Sclerosis, 15, 1175– 1182. [DOI] [PubMed] [Google Scholar]
- Kappos L., Polman C. H., Freedman M. S., Edan G., Hartung H. P., Miller D. H., Sandbrink R. (2006). Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology, 67, 1242– 1249. [DOI] [PubMed] [Google Scholar]
- Keegan B. M., Noseworthy J. H. (2002). Multiple sclerosis. Annual Review of Medicine, 53, 285– 302. [DOI] [PubMed] [Google Scholar]
- Kern S., Reichmann H., Ziemssen T. (2008). Adherence to neurologic treatment. Lessons from multiple sclerosis [article in German]. Nervenarzt, 79, 877– 882 [DOI] [PubMed] [Google Scholar]
- Lee W. C., Balu S., Cobden D., Joshi A. V., Pashos C. L. (2006). Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: An analysis of third-party managed care claims data. Clinical Therapeutics, 28, 1712– 1725. [DOI] [PubMed] [Google Scholar]
- Lugaresi A. (2009). Addressing the need for increased adherence to multiple sclerosis therapy: Can delivery technology enhance patient motivation? Expert Opinion on Drug Delivery, 6, 995– 1002. [DOI] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Bellomi F, Capra R., Rocca M., Filippi M. (2010). Neuropsychological rehabilitation in adult with multiple sclerosis. Neurological Sciences, 31 (Suppl 2), 271– 274. [DOI] [PubMed] [Google Scholar]
- Meyer-Moock S., Feng Y. Y. S., Dippel F. W., Kohlmann T. (2013). Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. ISPOR 16th Annual European Congress Dublin Ireland, 16 (7), A576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr D. C., Goodkin D. E., Likosky W., Gatto N., Neilley L. K., Griffin C., Stiebling B. (1996). Therapeutic expectations of patients with multiple sclerosis upon initiating interferon beta-1b: Relationship to adherence to treatment. Multiple Sclerosis, 2, 222– 226. [DOI] [PubMed] [Google Scholar]
- Novartis Pharma GmbH. (2009). Extavia (interferon beta-1b) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. [Google Scholar]
- Novartis Pharma GmbH. (2012). Extracare [Internet]. Retrieved from http://www.extracare.de/util/patienten-login.shtml
- Pascoe G. C. (1983). Patient satisfaction in primary health care: A literature review and analysis. Evaluation and Program Planning, 6, 185– 210. [DOI] [PubMed] [Google Scholar]
- Plosker G. L. (2011). Interferon-beta-1b: A review of its use in multiple sclerosis. CNS Drugs, 25 (1), 67– 88. [DOI] [PubMed] [Google Scholar]
- Polman C. H., Reingold S. C., Edan G., Filippi M., Hartung H. P., Kappos L., Wolinsky J. S. (2005). Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria” Annals of Neurology, 58, 840– 846. [DOI] [PubMed] [Google Scholar]
- Portaccio E., Amato M. P. (2009). Improving compliance with interferon-beta therapy in patients with multiple sclerosis. CNS Drugs, 23, 453– 462. [DOI] [PubMed] [Google Scholar]
- Portaccio E., Zipoli V., Siracusa G., Sorbi S., Amato M. P. (2008). Long-term adherence to interferon beta therapy in relapsing-remitting multiple sclerosis. European Neurology, 59, 131– 135. [DOI] [PubMed] [Google Scholar]
- Potagas C., Giogkaraki E., Koutsis G., Mandellos D., Tsirempolou E., Sfagos C., Vassilopoulos D. (2008). Cognitive impairment in different MS subtypes and clinically isolated syndromes. Journal of the Neurological Sciences, 267, 100– 106. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Oger J., Duquette P., Francis G. S., Belanger M., Laplante S., Grenier J. F. (1999). Treatment with interferon beta-1b improves quality of life in multiple sclerosis. Canadian Journal of Neurological Sciences, 26, 276– 282. [DOI] [PubMed] [Google Scholar]
- Ross A. P. (2008). Strategies for optimal disease management, adherence, and outcomes in multiple sclerosis patients. Neurology, 71, S1– S2. [DOI] [PubMed] [Google Scholar]
- The IFNB Multiple Sclerosis Study Group. (1993). Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology, 43, 655– 661. [DOI] [PubMed] [Google Scholar]
- Treadaway K., Cutter G., Salter A., Lynch S., Simsarian J., Corboy J., Frohman E. M. (2009). Factors that influence adherence with disease-modifying therapy in MS. Journal of Neurology, 256, 568– 576. [DOI] [PubMed] [Google Scholar]
- Tremlett H. L., Oger J. (2003). Interrupted therapy: Stopping and switching of the beta-interferons prescribed for MS. Neurology, 61, 551– 554. [DOI] [PubMed] [Google Scholar]
- Turner A. P., Williams R. M, Sloan A. P., Haselkorn J. K. (2009). Injection anxiety remains a long-term barrier to medication adherence in multiple sclerosis. Rehabilitation Psychology, 54, 116– 121. [DOI] [PubMed] [Google Scholar]


