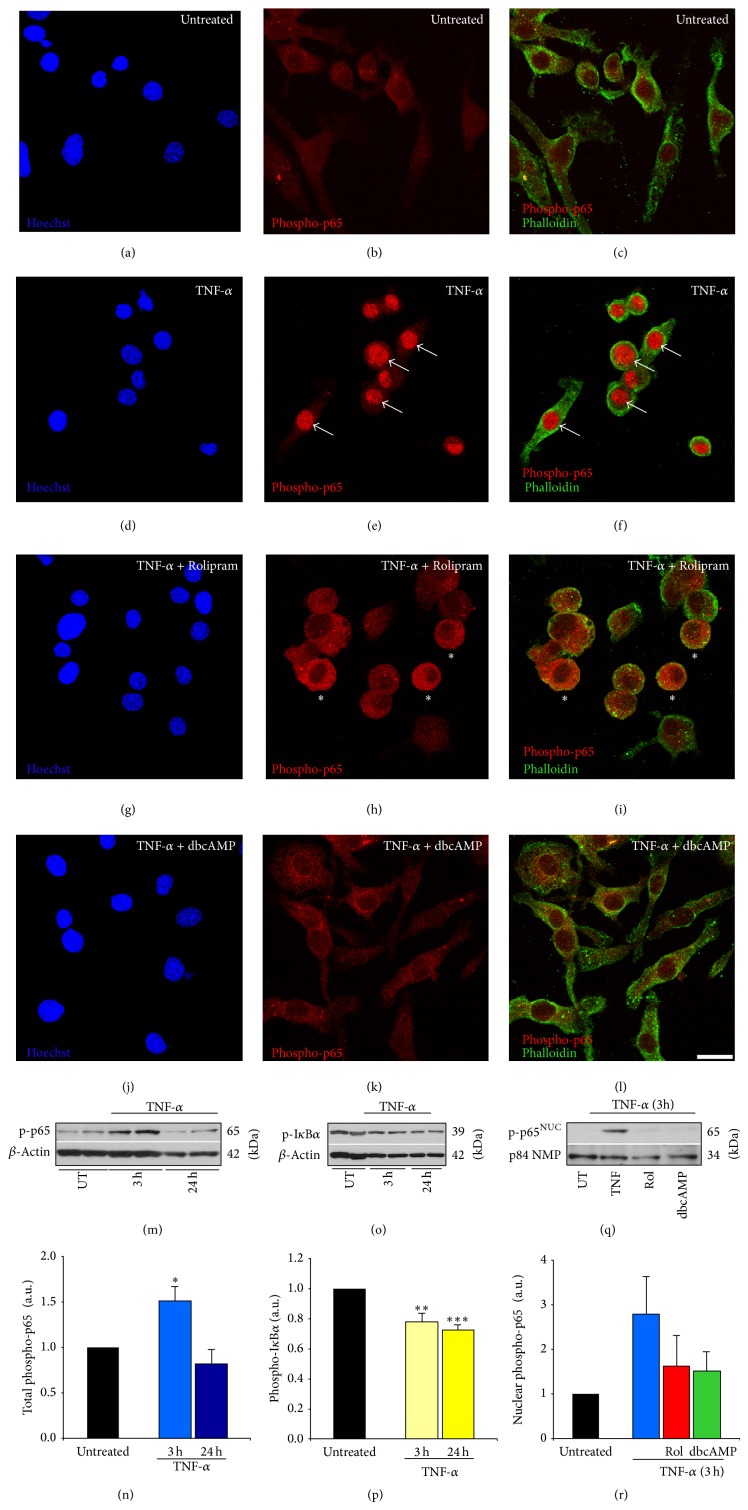
Figure 5.
Elevating cyclic AMP levels in TNF-α activated microglia using the PDE4 inhibitor Rolipram or a cyclic AMP analog inhibits nuclear translocation of p-p65Ser-536. Resting EOC2 microglial cells (a–c) showed very little p-p65Ser-536 immunoreactivity (red). Within 3 h of TNF-α stimulation, robust nuclear immunoreactivity for p-p65Ser-536 was observed (d–f). Treatment of TNF-α activated cells with either the PDE4 inhibitor Rolipram (g–i) or the cyclic AMP analogue db-cyclic AMP (j–l) significantly reduced nuclear p-p65Ser-536 immunoreactivity, with p-p65Ser-536 appearing to have been sequestered in the cytoplasm. Cell morphology is demarcated by staining with Alexa 488-tagged phalloidin (green) and the nuclear dye Hoechst (blue). Scale bar = 30 μm. White arrows and white asterisks show pronounced p-p65Ser-536 immunoreactivity within the nuclear and cytoplasmic compartments, respectively, among TNF-α stimulated microglia and TNF-α stimulated microglia with Rolipram. Immunoblotting of EOC2 microglial cell lysates showed significantly elevated levels of p-p65Ser-536 at 3 h following stimulation with TNF-α (m), which had returned to normal levels by 24 h. Quantification of p-p65Ser-536 levels by densitometry analysis (arbitrary units) normalized to β-actin (n). Immunoblotting for p-IκBα following TNF-α showed a persistent decrease through 24 h in EOC2 microglial cells (o). Quantification of p-IκBα levels by densitometry analysis (arbitrary units) normalized to β-actin (p). Western blot analysis of the purified nuclear fraction showed a trend towards elevated levels of nuclear p-p65Ser-536 following TNF-α, which did not occur in the presence of cyclic AMP elevating agents Rolipram or db-cyclic AMP (q). Quantification of nuclear p-p65Ser-536 levels by densitometry analysis (arbitrary units) normalized to p-84 nuclear matrix protein (r) revealed a trend for a cyclic AMP effect on nuclear p-p65Ser-536 after TNF-α, though no statistical significance was indicated. The results shown are the averages from four independent culture plate replicates for each treatment condition; the bands from up to two of the replicates are presented on the blot images. Errors are given as SEMs. Statistical significance to naive controls is indicated at, * P < 0.05, ** P < 0.01, or *** P < 0.001.