Table 1.
Overview of Two RCTs of ADT With or Without RT
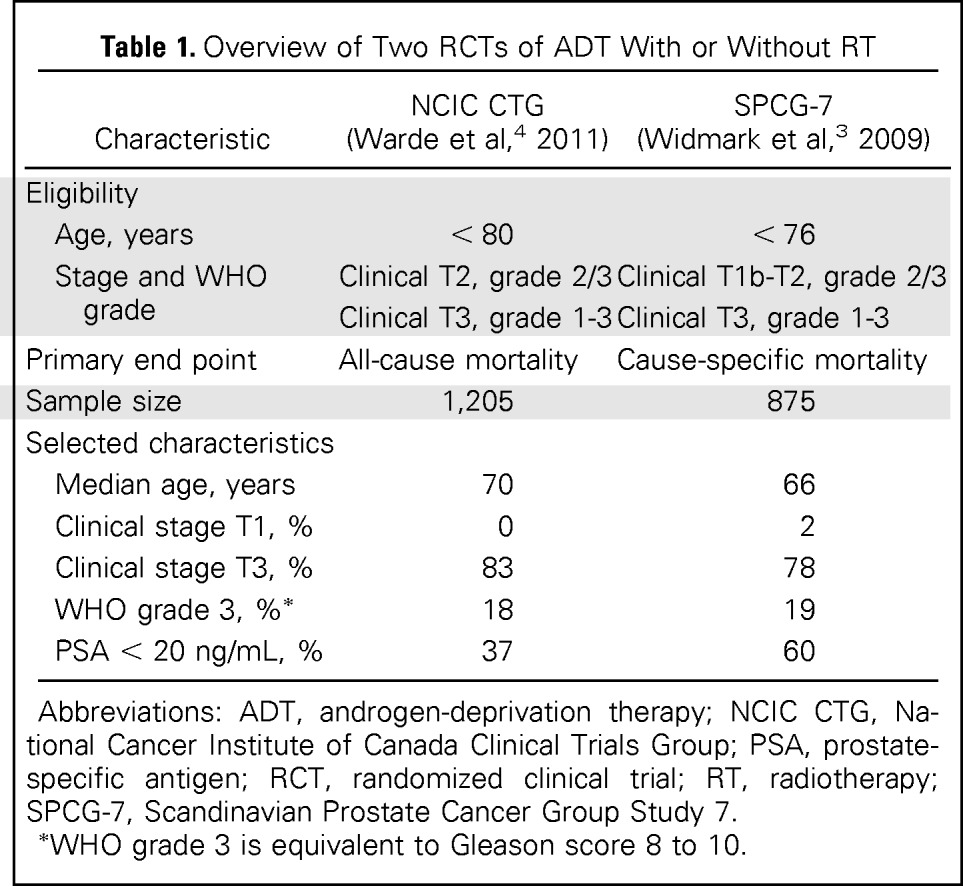
| Characteristic | NCIC CTG (Warde et al,4 2011) | SPCG-7 (Widmark et al,3 2009) |
|---|---|---|
| Eligibility | ||
| Age, years | < 80 | < 76 |
| Stage and WHO grade | Clinical T2, grade 2/3 | Clinical T1b-T2, grade 2/3 |
| Clinical T3, grade 1-3 | Clinical T3, grade 1-3 | |
| Primary end point | All-cause mortality | Cause-specific mortality |
| Sample size | 1,205 | 875 |
| Selected characteristics | ||
| Median age, years | 70 | 66 |
| Clinical stage T1, % | 0 | 2 |
| Clinical stage T3, % | 83 | 78 |
| WHO grade 3, %* | 18 | 19 |
| PSA < 20 ng/mL, % | 37 | 60 |
Abbreviations: ADT, androgen-deprivation therapy; NCIC CTG, National Cancer Institute of Canada Clinical Trials Group; PSA, prostate-specific antigen; RCT, randomized clinical trial; RT, radiotherapy; SPCG-7, Scandinavian Prostate Cancer Group Study 7.
WHO grade 3 is equivalent to Gleason score 8 to 10.
