Table 1.
Patient Demographics and Clinical Characteristics for PRO Participants Compared With All RESORT Participants
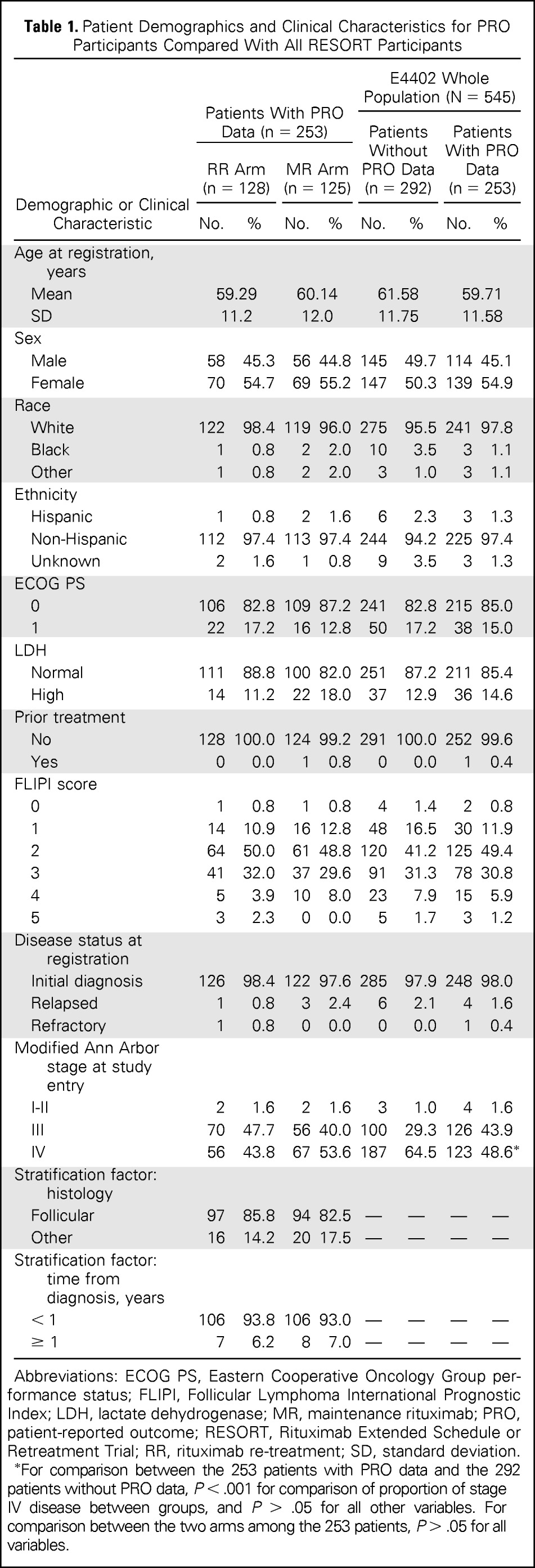
| Demographic or Clinical Characteristic | Patients With PRO Data (n = 253) |
E4402 Whole Population (N = 545) |
||||||
|---|---|---|---|---|---|---|---|---|
| RR Arm (n = 128) |
MR Arm (n = 125) |
Patients Without PRO Data (n = 292) |
Patients With PRO Data (n = 253) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Age at registration, years | ||||||||
| Mean | 59.29 | 60.14 | 61.58 | 59.71 | ||||
| SD | 11.2 | 12.0 | 11.75 | 11.58 | ||||
| Sex | ||||||||
| Male | 58 | 45.3 | 56 | 44.8 | 145 | 49.7 | 114 | 45.1 |
| Female | 70 | 54.7 | 69 | 55.2 | 147 | 50.3 | 139 | 54.9 |
| Race | ||||||||
| White | 122 | 98.4 | 119 | 96.0 | 275 | 95.5 | 241 | 97.8 |
| Black | 1 | 0.8 | 2 | 2.0 | 10 | 3.5 | 3 | 1.1 |
| Other | 1 | 0.8 | 2 | 2.0 | 3 | 1.0 | 3 | 1.1 |
| Ethnicity | ||||||||
| Hispanic | 1 | 0.8 | 2 | 1.6 | 6 | 2.3 | 3 | 1.3 |
| Non-Hispanic | 112 | 97.4 | 113 | 97.4 | 244 | 94.2 | 225 | 97.4 |
| Unknown | 2 | 1.6 | 1 | 0.8 | 9 | 3.5 | 3 | 1.3 |
| ECOG PS | ||||||||
| 0 | 106 | 82.8 | 109 | 87.2 | 241 | 82.8 | 215 | 85.0 |
| 1 | 22 | 17.2 | 16 | 12.8 | 50 | 17.2 | 38 | 15.0 |
| LDH | ||||||||
| Normal | 111 | 88.8 | 100 | 82.0 | 251 | 87.2 | 211 | 85.4 |
| High | 14 | 11.2 | 22 | 18.0 | 37 | 12.9 | 36 | 14.6 |
| Prior treatment | ||||||||
| No | 128 | 100.0 | 124 | 99.2 | 291 | 100.0 | 252 | 99.6 |
| Yes | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 1 | 0.4 |
| FLIPI score | ||||||||
| 0 | 1 | 0.8 | 1 | 0.8 | 4 | 1.4 | 2 | 0.8 |
| 1 | 14 | 10.9 | 16 | 12.8 | 48 | 16.5 | 30 | 11.9 |
| 2 | 64 | 50.0 | 61 | 48.8 | 120 | 41.2 | 125 | 49.4 |
| 3 | 41 | 32.0 | 37 | 29.6 | 91 | 31.3 | 78 | 30.8 |
| 4 | 5 | 3.9 | 10 | 8.0 | 23 | 7.9 | 15 | 5.9 |
| 5 | 3 | 2.3 | 0 | 0.0 | 5 | 1.7 | 3 | 1.2 |
| Disease status at registration | ||||||||
| Initial diagnosis | 126 | 98.4 | 122 | 97.6 | 285 | 97.9 | 248 | 98.0 |
| Relapsed | 1 | 0.8 | 3 | 2.4 | 6 | 2.1 | 4 | 1.6 |
| Refractory | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| Modified Ann Arbor stage at study entry | ||||||||
| I-II | 2 | 1.6 | 2 | 1.6 | 3 | 1.0 | 4 | 1.6 |
| III | 70 | 47.7 | 56 | 40.0 | 100 | 29.3 | 126 | 43.9 |
| IV | 56 | 43.8 | 67 | 53.6 | 187 | 64.5 | 123 | 48.6* |
| Stratification factor: histology | ||||||||
| Follicular | 97 | 85.8 | 94 | 82.5 | — | — | — | — |
| Other | 16 | 14.2 | 20 | 17.5 | — | — | — | — |
| Stratification factor: time from diagnosis, years | ||||||||
| < 1 | 106 | 93.8 | 106 | 93.0 | — | — | — | — |
| ≥ 1 | 7 | 6.2 | 8 | 7.0 | — | — | — | — |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FLIPI, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase; MR, maintenance rituximab; PRO, patient-reported outcome; RESORT, Rituximab Extended Schedule or Retreatment Trial; RR, rituximab re-treatment; SD, standard deviation.
For comparison between the 253 patients with PRO data and the 292 patients without PRO data, P < .001 for comparison of proportion of stage IV disease between groups, and P > .05 for all other variables. For comparison between the two arms among the 253 patients, P > .05 for all variables.
