Figure 3.
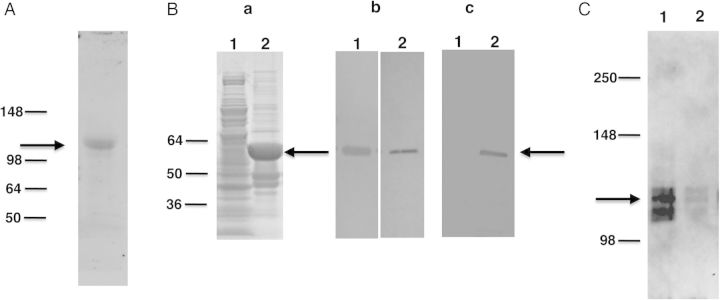
Western blot analysis of P. carinii Eng. A, Protein extracts prepared from partially purified P. carinii organisms probed with anti-Eng antibody. Immunoreactivity to an approximately 105 kDa band (indicated by arrow) was detected. B, Recombinant P. carinii Eng expressed in E. coli as a His-tag fusion protein was analyzed by SDS-PAGE and Western blotting. (Panel a) Recombinant protein was analyzed by SDS-PAGE gel stained with Coomassie Blue. Extracts from bacterial cells expressing recombinant protein showed a band of the expected size (approximately 60 kDa) indicated by the arrow (lane 2). This band was not seen with bacterial cells transformed with vector alone (lane 1). (Panel b) Western blot analysis of the bacterial cells expressing Eng protein, showing an immunoreactive band of the expected size when probed with anti-Eng antibody (lane 1) and anti-His tag antibody (lane 2). (Panel c) Western blot analysis of purified, refolded recombinant protein. An approximately 60 kDa band was detected with anti-His tag antibody (lane 2), but no immunoreactivity was observed in the vector-alone control (lane 1). C, The refolded recombinant Eng protein was tested for its ability to release Pneumocystis Msg. P. carinii organisms were incubated with the refolded protein or negative control sample. The supernatant obtained after centrifugation was analyzed for the presence of Msg by Western blot using a monoclonal antibody against P. carinii Msg. Strong immunoreactivity for Msg (approximately 115 kDa, shown by arrow) was seen in supernatant digested with recombinant Eng protein (lane 1). Only a weak signal was detected in supernatant incubated with protein preparation from the vector alone control (lane 2) or with buffer alone (data not shown). Abbreviations: Msg, major surface glycoprotein; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
