Abstract
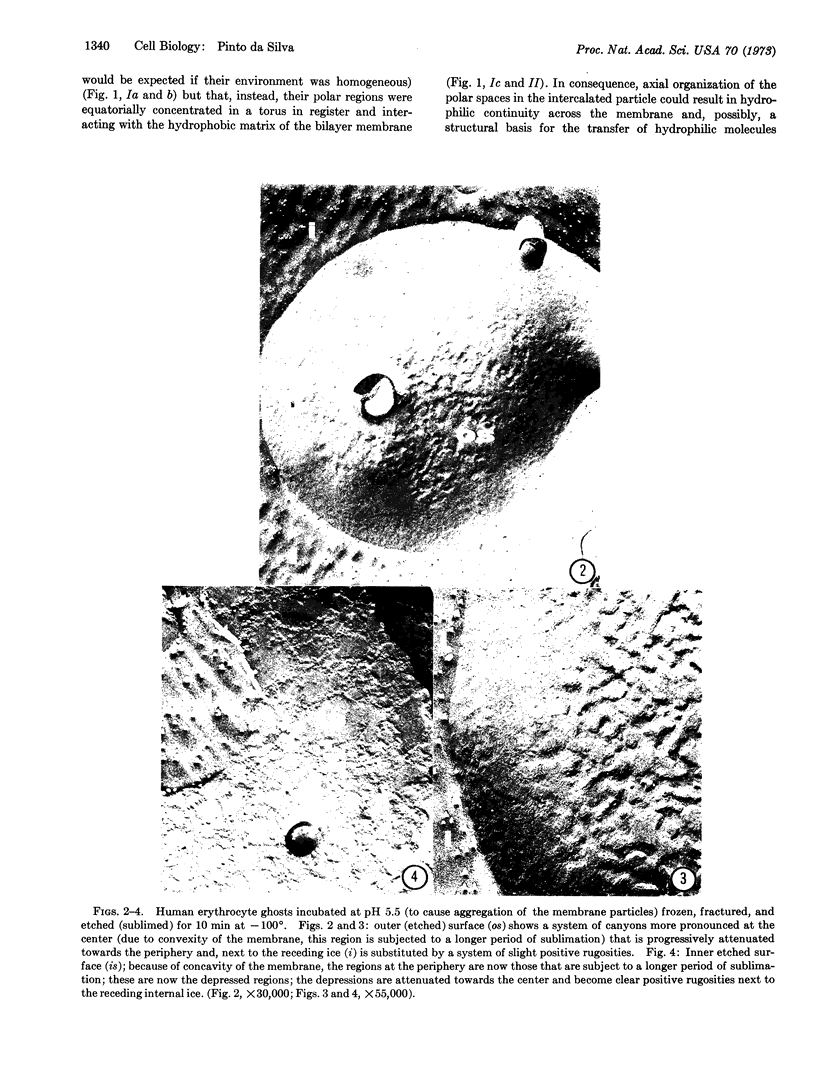
Although hydrophilic pores have been inferred to account for characteristics of the movement of water molecules across erythrocyte membranes, no direct evidence has associated such pores with actual structural differentiations within the membrane. Freeze-fracture and freeze-etch studies of isolated erythrocyte membranes show that they are comprised of fluid bilayer domains tranversed by protein-containing intercalations (“membrane intercalated particles”). It has previously been hypothesized that the topology of the polar and apolar spaces of the membrane-intercalated particles was not concentric the hydrophobic spaces being equatorially distributed. In consequence, axial organization of the hydrophilic regions could result in hydrophilic continuity across the membrane and might provide a structural basis for passage of hydrophilic molecules. The present experiments support this hypothesis. It is shown that sublimation at -100° of erythrocyte membrane suspensions (that had been incubated at pH 5.5 to cause aggregation of the membrane particles) results in progressive and selective sinking of the membrane regions comprised of aggregates of intercalated particles, i.e., that sublimation of water molecules occurs preferentially across these membrane regions. These results indicate that, under these experimental conditions, the membrane-intercalated particles provide a preferential structural pathway for passage of water molecules across erythrocyte ghost membranes.
Keywords: membrane proteins, transport, membrane structure, pore, electron microscopy
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W. W., Garan H., Berg H. C. Proteins of the human erythrocyte membrane as modified by pronase. J Mol Biol. 1971 Jun 28;58(3):783–797. doi: 10.1016/0022-2836(71)90040-4. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen myelin. Exp Cell Res. 1967 Mar;45(3):703–707. doi: 10.1016/0014-4827(67)90175-9. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Gary-Bobo C. M., DiPolo R., Solomon A. K. Role of hydrogen-bonding in nonelectrolyte diffusion through dense artificial membranes. J Gen Physiol. 1969 Sep;54(3):369–382. doi: 10.1085/jgp.54.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary-Bobo C. M., Solomon A. K. Effect of geometrical and chemical constraints on water flux across artificial membranes. J Gen Physiol. 1971 May;57(5):610–622. doi: 10.1085/jgp.57.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula N. B., Branton D., Satir P. The septate junction: a structural basis for intercellular coupling. Proc Natl Acad Sci U S A. 1970 Sep;67(1):213–220. doi: 10.1073/pnas.67.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula N. B., Reeves O. R., Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972 Feb 4;235(5336):262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- Holz R., Finkelstein A. The water and nonelectrolyte permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970 Jul;56(1):125–145. doi: 10.1085/jgp.56.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny G. M. Evidence for transfer of macromolecular RNA between mammalian cells in culture. Exp Cell Res. 1971 Apr;65(2):313–324. doi: 10.1016/0014-4827(71)90007-3. [DOI] [PubMed] [Google Scholar]
- PAGANELLI C. V., SOLOMON A. K. The rate of exchange of tritiated water across the human red cell membrane. J Gen Physiol. 1957 Nov 20;41(2):259–277. doi: 10.1085/jgp.41.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passow H. Passive ion permeability of the erythrocyte membrane. Prog Biophys Mol Biol. 1969;19(2):423–467. [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane intercalated particles: the plasma membrane as a planar fluid domain. Chem Phys Lipids. 1972 May;8(4):265–278. doi: 10.1016/0009-3084(72)90054-0. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Gilula N. B. Gap junctions in normal and transformed fibroblasts in culture. Exp Cell Res. 1972;71(2):393–401. doi: 10.1016/0014-4827(72)90309-6. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P. Translational mobility of the membrane intercalated particles of human erythrocyte ghosts. pH-dependent, reversible aggregation. J Cell Biol. 1972 Jun;53(3):777–787. doi: 10.1083/jcb.53.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Yee A. G., Hudspeth A. J. Gap junctions between electrotonically coupled cells in tissue culture and in brown fat. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2924–2927. doi: 10.1073/pnas.68.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B., Gitler C. Microviscosity of the cell membrane. Biochim Biophys Acta. 1972 Oct 23;288(1):231–236. doi: 10.1016/0005-2736(72)90242-8. [DOI] [PubMed] [Google Scholar]
- Sandri C., Akert K., Livingston R. B., Moor H. Particle aggregations at specialized sites in freeze-etched postsynaptic membranes. Brain Res. 1972 Jun 8;41(1):1–16. doi: 10.1016/0006-8993(72)90612-9. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., Gary-Bobo C. M., Solomon A. K. Permeability of red cell membranes to small hydrophilic and lipophilic solutes. J Gen Physiol. 1971 Sep;58(3):238–258. doi: 10.1085/jgp.58.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Solomon A. K. Characterization of biological membranes by equivalent pores. J Gen Physiol. 1968 May;51(5 Suppl):335S+–335S+. [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., van Deenen L. L., Elbers P. F. Phase transitions of phospholipid bilayers and membranes of Acholeplasma laidlawii B visualized by freeze fracturing electron microscopy. Biochim Biophys Acta. 1972 Nov 2;288(2):326–332. doi: 10.1016/0005-2736(72)90253-2. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Zahler P. H. Protein conformations in cellular membranes. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1552–1559. doi: 10.1073/pnas.56.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]





