FIGURE 3.
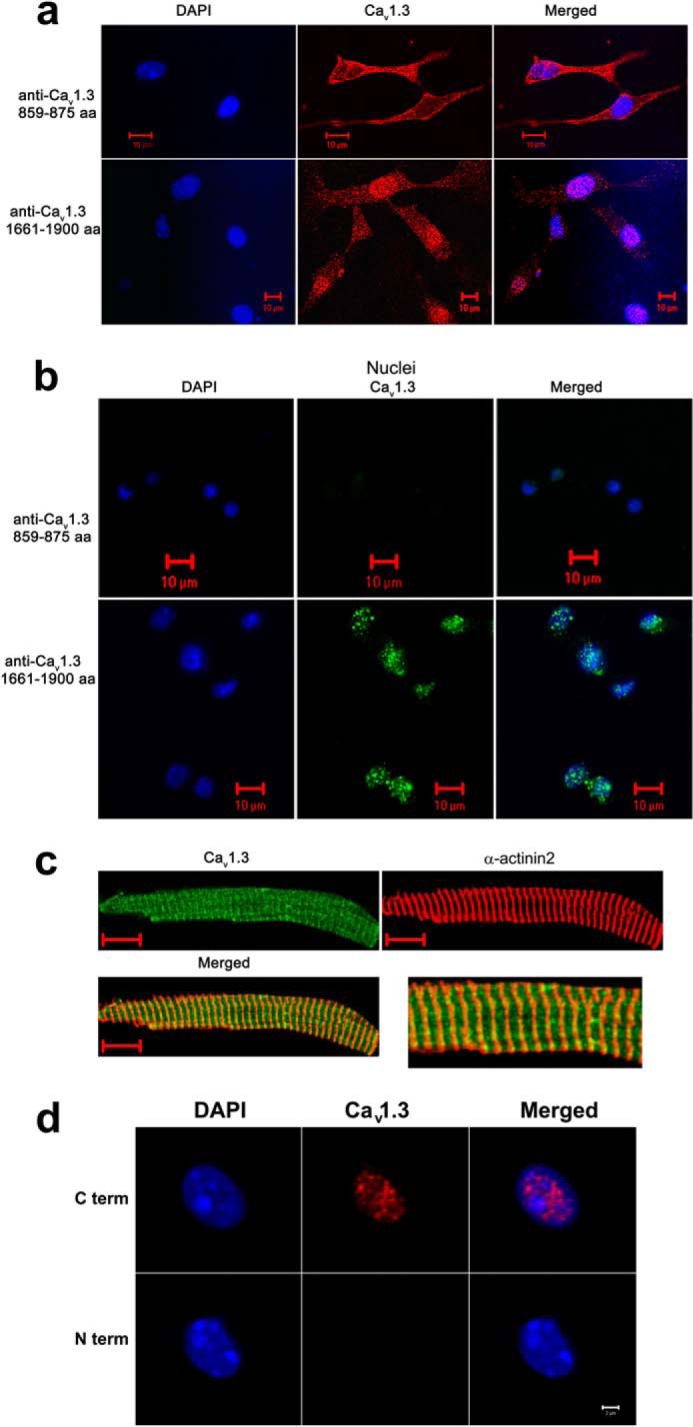
The C terminus of the Cav1.3 channel is localized in the nucleus of cardiac myocytes from wild-type mice. a, immunofluorescence confocal microscopic images of neonatal mouse cardiomyocytes stained using an antibody recognizing an epitope in the II-III cytoplasmic loop of the Cav1.3 channel (amino acids 859–875, loop antibody, upper panels). No detectable nuclear fluorescence is observed. In contrast, a high nuclear fluorescence signal is detected when an antibody that recognizes the C terminus of Cav1.3 (amino acids 1661–1990) is used (lower panels). b, isolated nuclei from adult atrial myocytes. The C-terminal antibody of the Cav1.3 channel detects a high nuclear fluorescence in contrast to the antibody directed against the II-III cytoplasmic loop. Nuclei were stained using DAPI (blue). c, adult atrial myocytes co-stained with anti-Cav1.3 (loop antibody, red) and α-actinin2 (green) antibodies. Scale bars are 10 μm. Right panel shows the merged image at higher magnification. d, isolated nuclei from adult atrial myocytes. Two different monoclonal antibodies for the Cav1.3 Ca2+ channel were used including: 1) anti-Cav1.3 Ca2+ channel (NeuroMab clone L48A/9) directed against amino acids 859–875 in the N terminus of rat Cav1.3, and 2) anti-Cav1.3 Ca2+ channel (NeuroMab clone N38/8) directed against amino acids 2025–2161 in the C terminus of rat Cav1.3. The monoclonal C-terminal antibody of Cav1.3 channel (C term) detects a high nuclear fluorescence in contrast to the monoclonal N-terminal antibody (N term). Nuclei were stained using DAPI (blue). Scale bar is 2 μm. The experiments were repeated independently three times.
