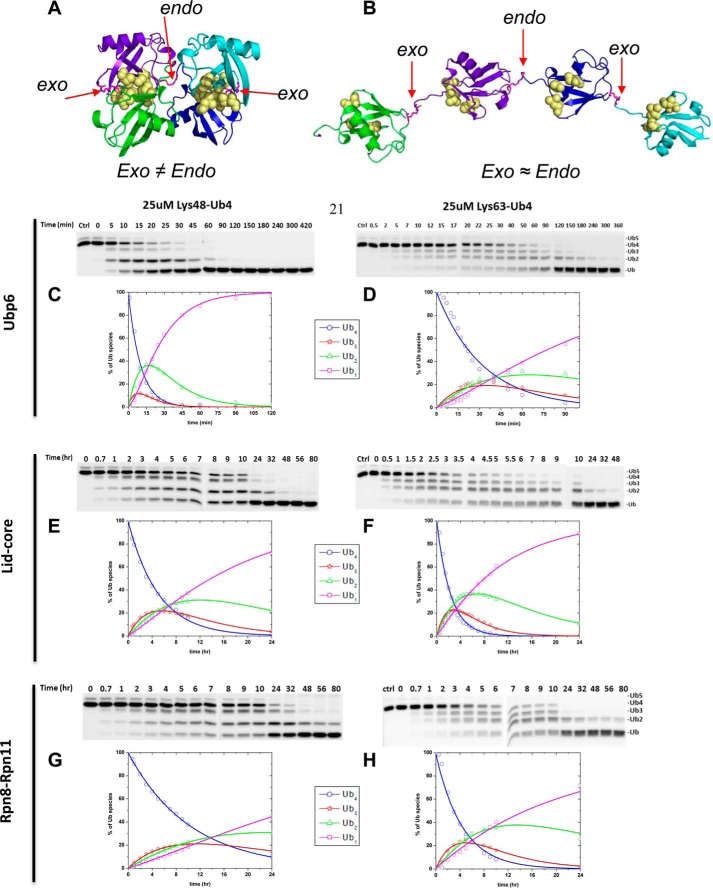
FIGURE 4.
A, the tetrameric form of Lys48-Ub4 adopts a highly compact structural conformation at physiological conditions (100). B, in contrast, the extended conformation of tetrameric Lys63-Ub4 similarly exposes all Lys63 linkages (101). The positions of the isopeptide bonds are indicated with red arrows. The Leu8, Ile44, and Val70 hydrophobic patch of Ub is represented as yellow spheres. Ub4 disassembly was quantified by in-gel fluorescence. Ubp6 (C and D), lid-core (E and F), and Rpn8·Rpn11 heterodimer (G and H) were incubated with Alexa Fluor 488-labeled Lys48-Ub4 chains (left) or Lys63-Ub4 (right). The percentage of each Ub species calculated from contribution to total fluorescence is displayed graphically. Experimental data points are plotted as symbols along with the curves representing the results of mathematical analysis (Equations 2–5); the results for Ub4 species are shown in blue, Ub3 results are in red, Ub2 results are in green, and Ub1 results are in magenta. The total florescence signal integrated from contributions of mono-, di-, tri-, and tetra-Ub remained constant throughout the time course with less than 5% S.D.