FIGURE 3.
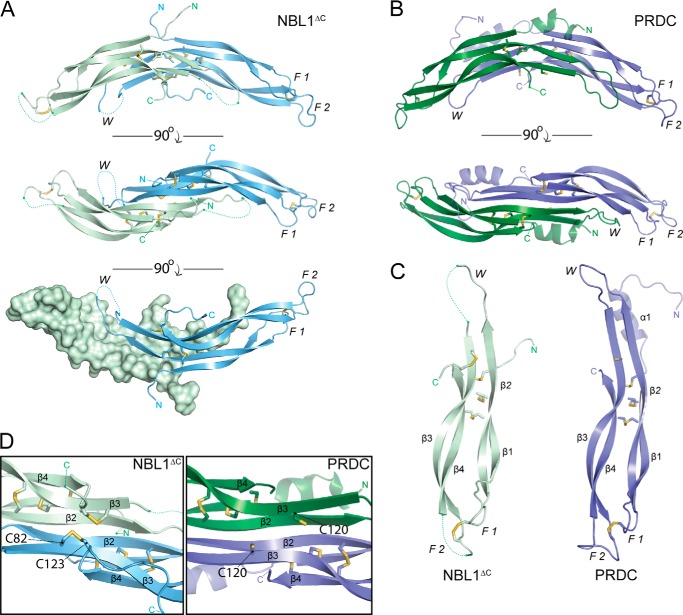
Crystal structure of NBL1ΔC. A, ribbon representation of the NBL1ΔC dimer with the Chain A monomer shown in pale green and Chain B monomer shown in pale blue. Sticks represent disulfide bonds with sulfurs colored yellow-orange. Dotted lines represent areas that cannot be resolved in the electron density and connect amino acids according to the primary protein sequence. Different views (from top to bottom) show the dimer rotated 90° about the horizontal axis. F1, finger 1; F2, finger 2; W, wrist region. B, ribbon representation of the PRDC dimer crystal structure (Protein Data Bank code 4JPH, Ref. 16). C, comparison of the NBL1ΔC and PRDC monomer structures. β-Strands are labeled in each monomer (β1-β4) in order from the N terminus. D, view of the bottom, concave surfaces of NBL1ΔC (left) and PRDC (right), highlighting their cystine knots. As can be seen, the fifth disulfide bond in NBL1ΔC links the final cysteine of the protein to the synonymous free cysteine in PRDC and reiterates that these proteins do not form covalently attached dimers.