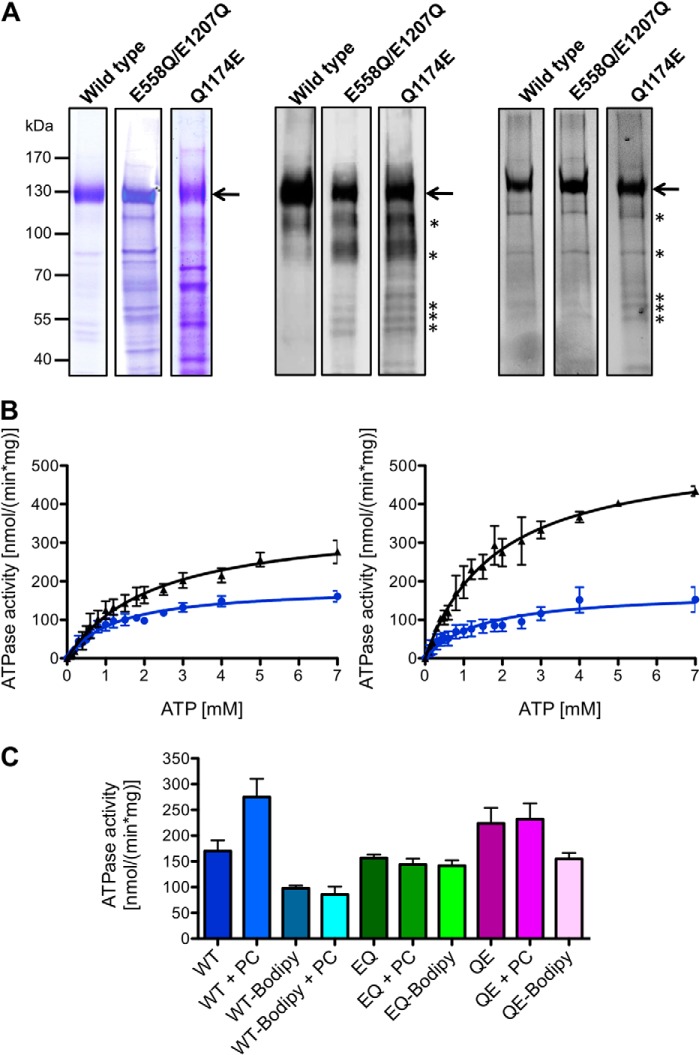
FIGURE 1.
A, human wild type MDR3, the E558Q/E1207Q double mutant, and the Q1174E mutant purified from P. pastoris. The MDR3 variants containing a C-terminal His6 tag and a calmodulin-binding peptide were expressed in the yeast P. pastoris and purified as described under “Experimental Procedures.” 10 μg of purified MDR3 was resolved on a 7% SDS-PAGE and either stained with Coomassie Brilliant Blue (left panel) or detected by immunoblotting (middle panel) using the monoclonal anti-P-gp C219 antibody. MDR3 was cross-linked by the thiol-reactive maleimide-BODIPY fluorophore (MDR3-BODIPY) and analyzed by fluorescence imaging using excitation wavelength at 488 nm and emission wavelength at 520 nm (right panel). Molecular mass markers are shown on the left. MDR3 is indicated with an arrow, and degradation products of MDR3 are shown with asterisks. B, ATPase activity of purified wild type MDR3 (black triangle) and MDR3-BODIPY (blue circles) in the absence (left panel) or presence (right panel) of DOPC lipids. C, ATPase activity of purified wild type MDR3 (blue), the ATPase-deficient MDR3 EQ/EQ mutant (green), and the Q1174E mutant (magenta) and the corresponding cross-linked BODIPY derivatives in the presence of 2 mm ATP and 300 μm DOPC (PC).