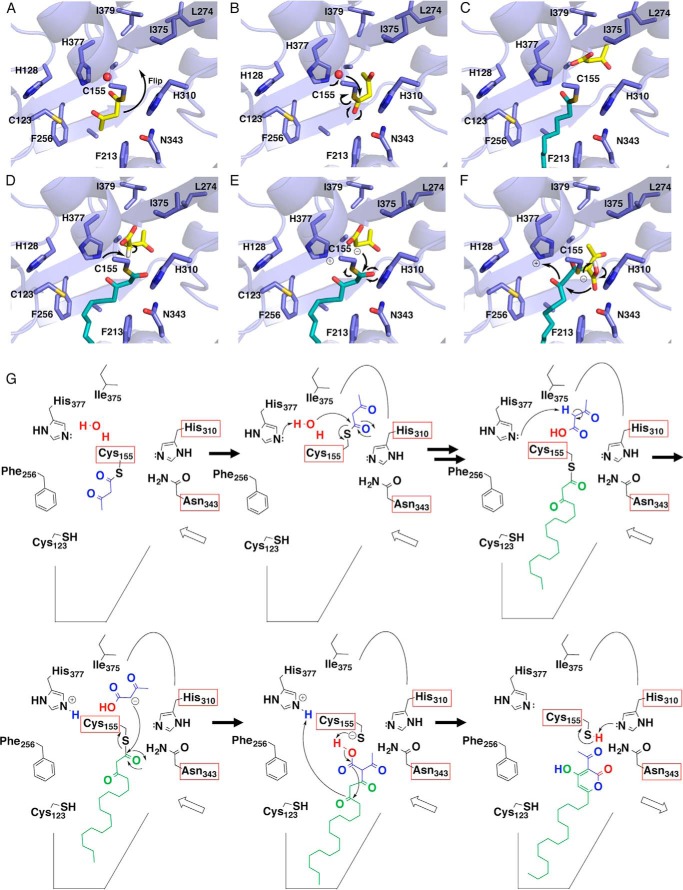
FIGURE 9.
Proposed mechanism for the CsyB enzymatic reaction. A–F, three-dimensional models of reorientation of the diketide intermediate covalently bound to the catalytic Cys155 (A), hydrolysis of the first β-ketoacyl diketide unit (B), loading of the fatty acyl-CoA onto the catalytic Cys-155 (C), proton abstraction from the stored β-ketoacyl diketide unit (D), coupling reaction between two β-ketoacyl units (E), and lactamization to produce the final products (F). G, schematic representation of the proposed mechanism.