Abstract
Intact rabbit reticulocyte cells synthesize two predominant species of membrane polypeptides; at least 10 reticulocyte membrane polypeptide species are not produced by the cells. Cell-free extracts of reticulocytes, free of any membranes or membrane-bound polyribosomes, synthesize large amounts of these two membrane polypeptides; one of these polypeptides appears to be modified, probably by loss of 20-40 amino acids, after it is incorporated into the membrane. Deprivation of hemin results in inhibition of synthesis by lysates of membrane proteins, globin, and all other cytoplasmic proteins.
Keywords: rabbit, hemin, globin, gel electrophoresis
Full text
PDF

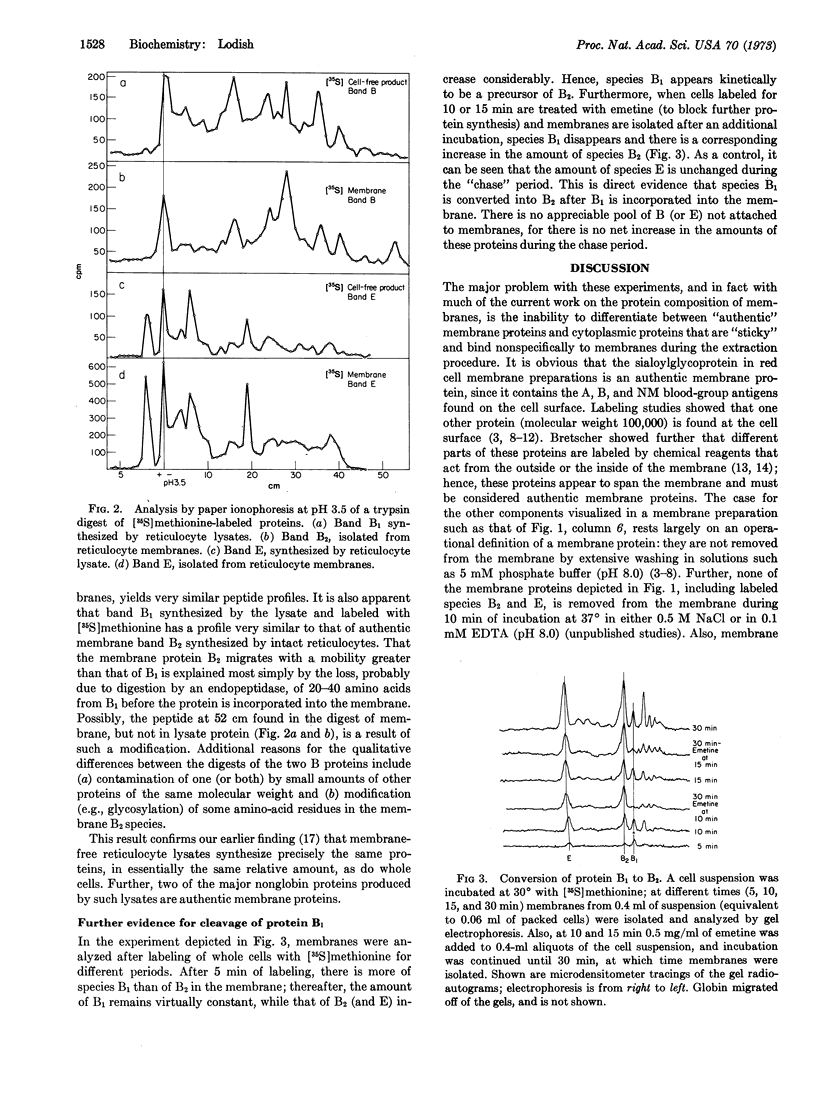


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Adamson S. D., Herbert E., Kemp S. F. Effects of hemin and other porphyrins on protein synthesis in a reticulocyte lysate cell-free system. J Mol Biol. 1969 Jun 14;42(2):247–258. doi: 10.1016/0022-2836(69)90041-2. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Human erythrocyte membranes: specific labelling of surface proteins. J Mol Biol. 1971 Jun 28;58(3):775–781. doi: 10.1016/0022-2836(71)90039-8. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Bulova S. I., Burka E. R. Biosynthesis of nonglobin protein by membrane-bound ribosomes in reticulocytes. J Biol Chem. 1970 Oct 10;245(19):4907–4912. [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti G. Membrane proteins. Annu Rev Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Studies on cessation of protein synthesis in a reticulocyte lysate cell-free system. Biochim Biophys Acta. 1970 Jul 16;213(1):237–240. doi: 10.1016/0005-2787(70)90028-6. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J. Protein components of erythrocyte membranes from different animal species. Biochemistry. 1970 Dec 8;9(25):5037–5040. doi: 10.1021/bi00827a032. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Jacobsen M. Regulation of hemoglobin synthesis. Equal rates of translation and termination of - and -globin chains. J Biol Chem. 1972 Jun 10;247(11):3622–3629. [PubMed] [Google Scholar]
- Lodish H. F. Secondary structure of bacteriophage f2 ribonucleic acid and the initiation of in vitro protein biosynthesis. J Mol Biol. 1970 Jun 28;50(3):689–702. doi: 10.1016/0022-2836(70)90093-8. [DOI] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Rabinovitz M. Evidence for an inhibitor in the control of globin synthesis by hemin in a reticulocyte lysate. Biochem Biophys Res Commun. 1969 Apr 10;35(1):79–85. doi: 10.1016/0006-291x(69)90485-9. [DOI] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K. An in vitro protein synthesizing system from mouse L fibroblasts infected with reovirus. Virology. 1971 Sep;45(3):724–733. doi: 10.1016/0042-6822(71)90186-3. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Hydroxylapatite chromatography of protein-sodium dodecyl sulfate complexes. A new method for the separation of polypeptide subunits. J Biol Chem. 1972 Aug 25;247(16):5194–5198. [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Position of glycoprotein polypeptide chain in the human erythrocyte membrane. FEBS Lett. 1971 Oct 15;18(1):95–97. doi: 10.1016/0014-5793(71)80416-7. [DOI] [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- Rosenberg S. A., Guidotti G. Fractionation of the protein components of human erythrocyte membranes. J Biol Chem. 1969 Oct 10;244(19):5118–5124. [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. 5. In vivo incorporation of leucine-1-C14 into the chymotrypsinogen of various cell fractions. J Biophys Biochem Cytol. 1960 Jul;7:619–630. doi: 10.1083/jcb.7.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Blobel G. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. II. Location of the polypeptides in rough microsomes. J Cell Biol. 1970 Apr;45(1):146–157. doi: 10.1083/jcb.45.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Trayer H. R., Nozaki Y., Reynolds J. A., Tanford C. Polypeptide chains from human red blood cell membranes. J Biol Chem. 1971 Jul 25;246(14):4485–4488. [PubMed] [Google Scholar]
- Warren L., Glick M. C. Membranes of animal cells. II. The metabolism and turnover of the surface membrane. J Cell Biol. 1968 Jun;37(3):729–746. doi: 10.1083/jcb.37.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzler R. J., Harris E. D., Pekas D. J., Johnson C. A., Weber P. Studies on glycopeptides released by trypsin from intact human erythrocytes. Biochemistry. 1967 Jul;6(7):2195–2202. doi: 10.1021/bi00859a042. [DOI] [PubMed] [Google Scholar]




