Figure 2. Fluorescence-activated flow sorting to enrich genetically modified DMD myoblasts.
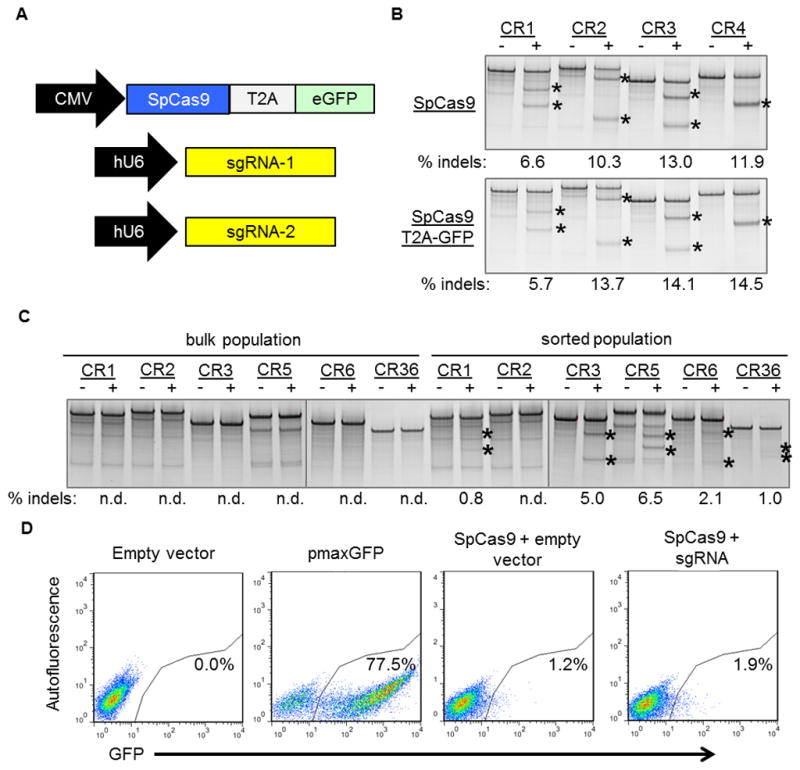
(A) A plasmid expressing a human-codon optimized SpCas9 protein linked to a GFP marker using a T2A ribosomal skipping peptide sequence was co-electroporated into human DMD myoblasts with one or two plasmids carrying sgRNA expression cassettes. (B) The indicated sgRNA expression cassettes were independently co-transfected into HEK293Ts with a separate plasmid expressing SpCas9 with (bottom) or without (top) a GFP marker linked to SpCas9 by a T2A ribosomal skipping peptide sequence. Gene modification frequencies were assessed at 3 days post-transfection by the Surveyor assay. (C) DMD myoblasts with deletions of exons 48–50 in the dystrophin gene were treated with sgRNAs that correct the dystrophin reading frame in these patient cells. Gene modification was assessed at 20 days post-electroporation in unsorted (bulk) or GFP+ sorted cells. (D) GFP expression in DMD myoblasts 3 days after electroporation with indicated expression plasmids. Transfection efficiencies and sorted cell populations are indicated by the gated region.
