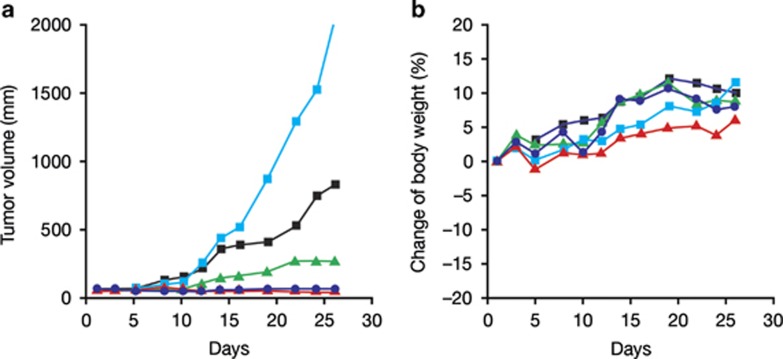
Figure 4.
Efficacy and tolerability of volasertib in human AML xenograft model. Nude mice bearing established subcutaneous MV4-11 AML tumors with an average size of ~65 mm3 were treated intravenously for 4 weeks with either vehicle (light blue squares) or volasertib at 40 mg/kg (blue circles), 20 mg/kg (green triangles), or 10 mg/kg once a week (black squares), or at 20 mg/kg two times a week on consecutive days (red triangles). Median tumor volumes of eight animals per treatment group (a) and median body weight change as % of initial body weight (b) are shown. Efficacy has also been demonstrated in three disseminated AML models (MV4-11 (FLT3-ITD/FLT3-ITD), Molm-13 (FLT3-ITD/wild-type FLT3) and THP-1 (wild-type FLT3/wild-type FLT3) cell lines).