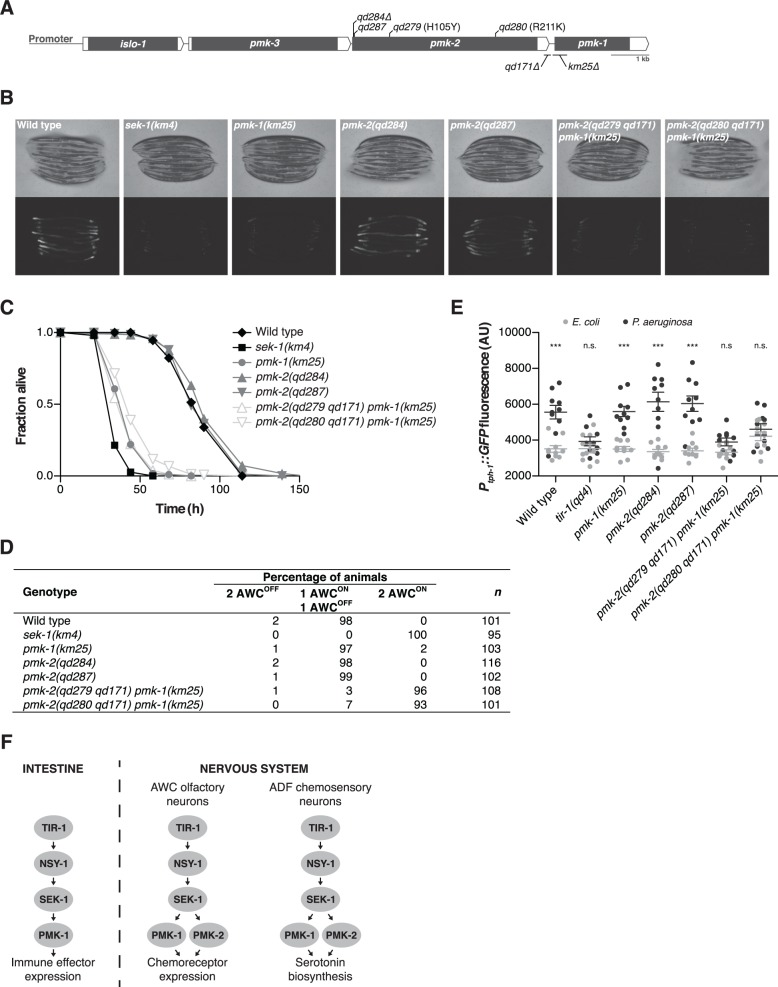
Fig 1. PMK-1 and PMK-2 function redundantly in the nervous system but not the intestine.
(A) The pmk operon showing mutations utilized and isolated in this study. Gray fill, corresponding unspliced transcript; white fill, corresponding 5’ and 3’UTRs. pmk-2 mutations: qd284, 10 bp deletion, frameshift; qd287, 7 bp insertion, frameshift; qd279 and qd280, as indicated in reference to isoform pmk-2b (release WS245); qd171, 913/184 bp insertion/deletion. pmk-1 mutation: km25, 375 bp deletion. (B-E) Phenotypic analysis of mutants deficient in p38 MAPK signaling. (B) Bright field and fluorescence microscopy images of 1-day-old adult worms carrying the agIs219[PT24B8.5::GFP] transgene. (C) Pathogenesis assay of L4 larval stage worms on P. aeruginosa PA14. All strains carry the agIs219[PT24B8.5::GFP] transgene. (D) Expression of str-2::GFP in the AWC olfactory neurons of L3 and L4 larval stage and young adult worms. (E) Quantification of GFP expression from the nIs145[P tph-1::GFP] transgene in 1-day-old adult worms after a 6 hr exposure to E. coli OP50 or P. aeruginosa PA14. Shown is a representative experiment. Error bars, ± standard deviation. (n.s. not significant, *** P<0.001, two-way ANOVA with Bonferroni post-test). (F) PMK-1 p38 MAPK functions independently of PMK-2 p38 MAPK in the intestine downstream of the TIR-1-NSY-1-SEK-1 signaling module in the regulation of immune effector gene expression in response to pathogenic microbes. PMK-1 and PMK-2 p38 MAPKs function redundantly in the nervous system downstream of TIR-1-NSY-1-SEK-1 in the regulation of AWC neural asymmetry and pathogen-induced upregulation of tph-1 expression in the ADF neurons.