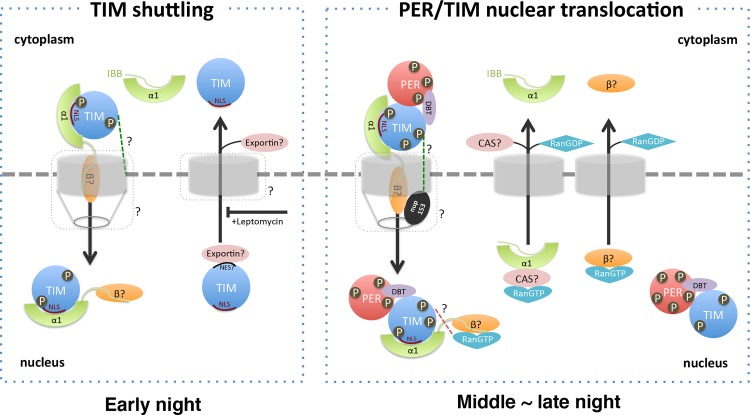
Figure 7. A model for nuclear translocation of Drosophila clock proteins, PER and TIM.
Left: In the early night, TIM shuttles in and out the nucleus. TIM is recognized by IMPα1 in the cytoplasm and translocated into the nucleus. The interaction between TIM and IMPα1 is regulated by phosphorylation of TIM. Nuclear entry of the TIM- IMPα1 complex may involve other components like Ran or NUP153 in the nuclear pore complex (green dotted line). In the nucleus, TIM binds to an unknown exportin and is transported back to the cytoplasm, a process that is inhibited by Leptomycin. Right: In the middle to late night, the TIM-IMPα1 complex transports PER into the nucleus, which then leads to the retention of TIM. Note that we cannot exclude the possibility that PER is also transported earlier in the night, but not retained in the nucleus. We speculate that at a specific time, TIM and/or PER is modified by phosphorylation events, which alters the conformation of the PER-TIM-IMPα1 complex and promotes nuclear expression of first PER and then TIM. The PER-TIM-IMPα1 complex binds to IMPβ and passes though the nuclear pore complex containing NUP153. In the nucleus, RanGTP binds to IMPβ, dissociating the complex and releasing the cargo proteins, PER and TIM (a possible direct interaction between TIM and RanGTP is indicated by a red dotted line). After its release from the importin machinery, PER retains TIM in the nucleus. As per the canonical nuclear entry mechanism, IMP α1 and β are likely recycled back to the cytoplasm by exportin Cas and RanGTP, respectively. Question marks indicate unknown processes or components that need to be experimentally validated.