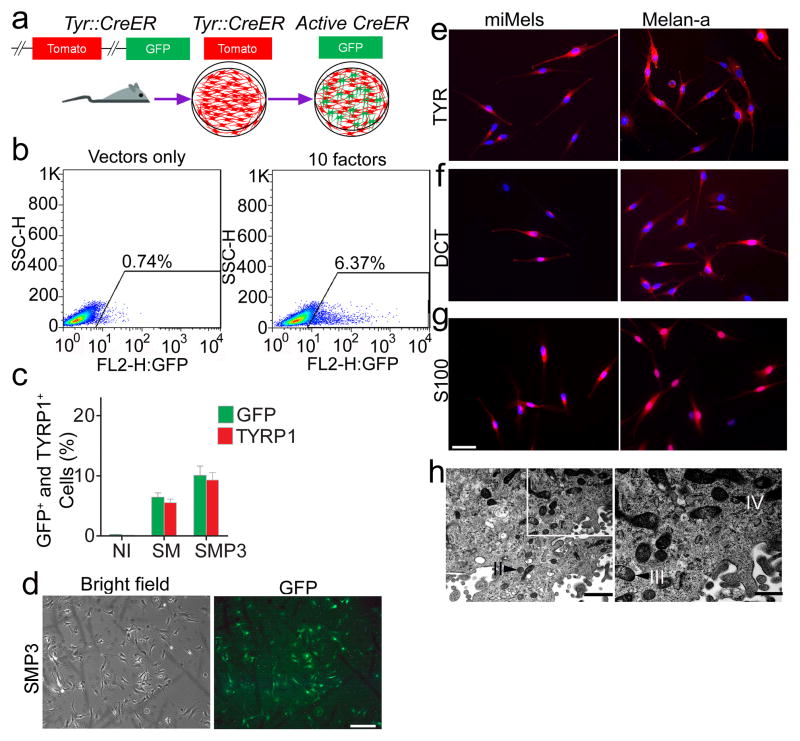
Figure 1. Screening for melanocyte direct reprogramming factors.
a. Scheme for melanocyte direct reprogramming transcription factor (TF) screening. Screening was performed using fibroblasts from the Tyrosinase-CreER/Gt(ROSA)26Sor tm4(ACTB-tdTomato,-EGFP)Luo/J mice. When tyrosinase (TYR) was activated, Cre activation resulted in excision of the tomato cassette and expression of GFP in the presence of 4-HT. b. Flow cytometric analysis of the GFP+ cells after cells were infected with viruses packaged with 10 candidate factors (right panel) or vector only (left panel). c. Flow cytometric analysis of GFP and TYRP1 expression after cells were infected with control vectors (NI), SOX10 and MITF (SM) or SOX10, MITF and PAX3 (SMP3). Representative data are from three independent experiments. d. Morphology of FACS sorted GFP+ cells. Mouse fibroblasts were infected with SMP3 and sorted based on GFP expression at Day 14. Scale bar indicates 50 μm. e–g. Immunostaining analysis of mouse induced mouse melanocytes (miMels) using antibodies specific for TYR (e), DCT (f) and S100 (g). Secondary antibody was labeled with Alexa Fluro 594. DAPI was used to stain the nuclei. Melan-a mouse melanocytes were used as a positive control. Scale bar indicates 30 μm. h. Electron microscopy analyses showed that miMels contained many mature melanosomes in the cytoplasm. Arrow heads point to the different stages of melanosomes, including stage II, III and IV. Scale bar indicates 400 nm (left panel) and 200 nm (right panel).