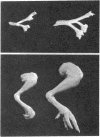
Abstract
The evolution of the cholinergic (nicotinic) receptor in chick muscles is monitored during embryonic development with a tritiated α-neurotoxin from Naja nigricollis and compared with the appearance of acetylcholinesterase. The specific activity of these two proteins reaches a maximum around the 12th day of incubation. By contrast, choline acetyltransferase reaches an early maximum of specific activity around the 7th day of development, and later continuously increases until hatching. Injection of α-toxin in the yolk sac at early stages of development causes an atrophy of skeletal and extrinsic ocular-muscles and of their innervation. In 16-day embryos treated by the α-toxin, the endplates revealed by the Koelle reaction are almost completely absent. The total content and specific activities of acetylcholinesterase and choline acetyltransferase in atrophic muscles are markedly reduced.
Keywords: cholinergic receptor, choline acetyltransferase, acetylcholinesterase, embryonic development of neuromuscular junction
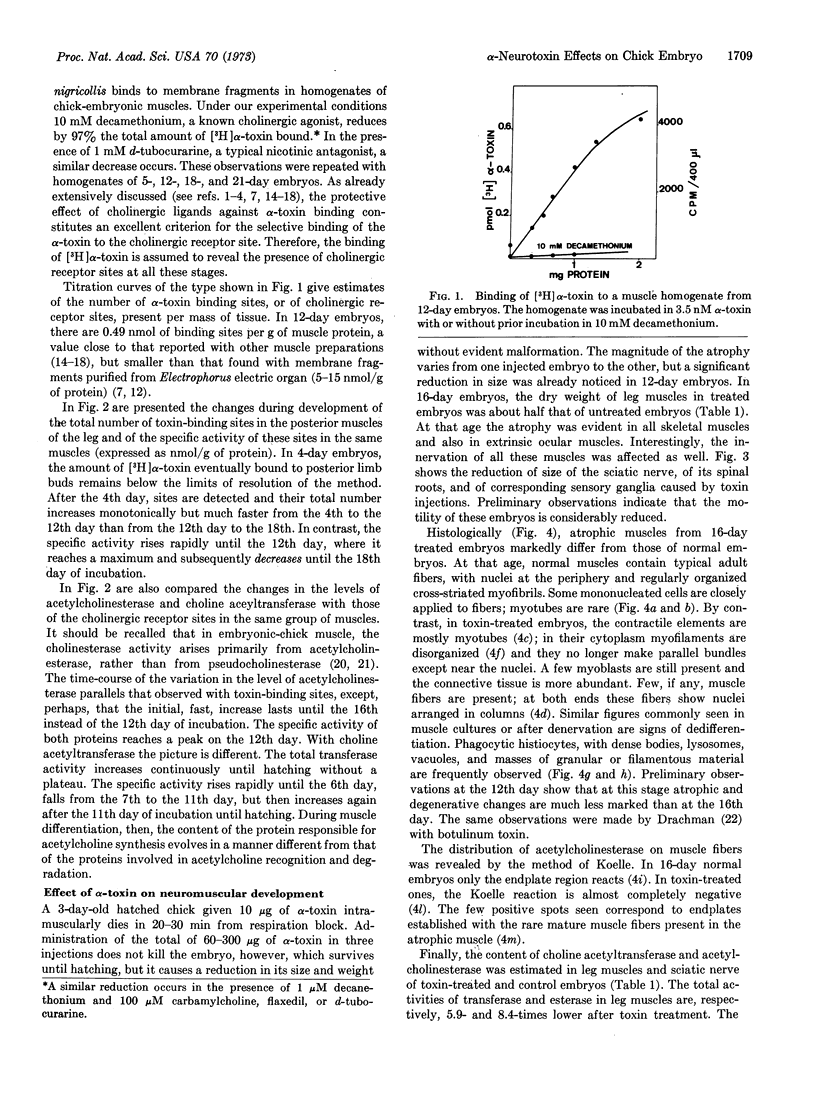
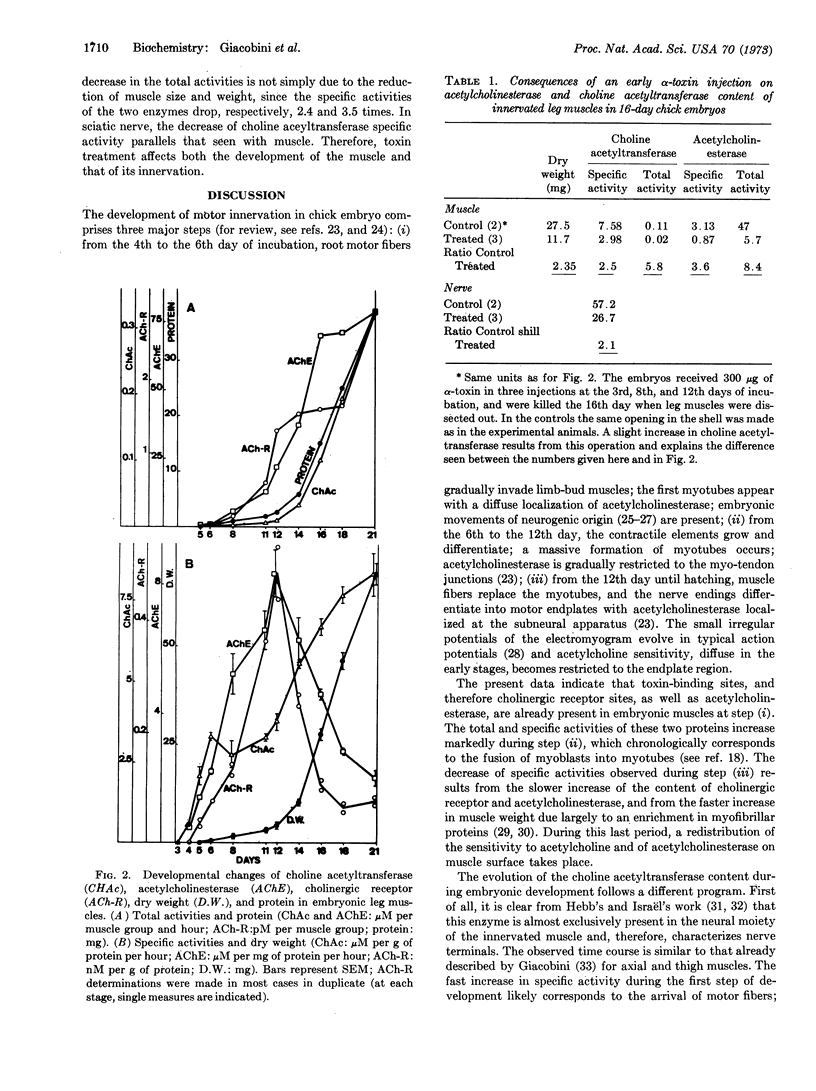
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alconero B. B. The nature of the earliest spontaneous activity of the chick embryo. J Embryol Exp Morphol. 1965 Jun;13(3):255–266. [PubMed] [Google Scholar]
- Baril E. F., Herrmann H. Studies of muscle development. II. Immunological and enzymatic properties and accumulation of chromatographically homogeneous myosin of the leg musculature of the developing chick. Dev Biol. 1967 Apr;15(4):318–333. doi: 10.1016/0012-1606(67)90030-9. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Wieckowski J., Chiu T. H. Cholinergic receptor molecules and cholinesterase molecules at mouse skeletal muscle junctions. Nature. 1971 Nov 26;234(5326):207–209. doi: 10.1038/234207a0. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Kelly R. B., Sargent P. B., Williamson P., Hall Z. W. Binding of -bungarotoxin to acetylcholine receptors in mammalian muscle (snake venom-denervated muscle-neonatal muscle-rat diaphragm-SDS-polyacrylamide gel electrophoresis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):147–151. doi: 10.1073/pnas.69.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Boëthius J. The development of the electromyogram in chick embryos. J Exp Zool. 1967 Aug;165(3):419–424. doi: 10.1002/jez.1401650309. [DOI] [PubMed] [Google Scholar]
- COUTEAUX R. THE DIFFERENTIATION OF SYNAPTIC AREAS. Proc R Soc Lond B Biol Sci. 1963 Nov 19;158:457–480. doi: 10.1098/rspb.1963.0058. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan W. M., Wenger E. Cell loss in the trochlear nucleus of the chick during normal development and after radical extirpation of the optic vesicle. J Exp Zool. 1967 Mar;164(2):267–280. doi: 10.1002/jez.1401640210. [DOI] [PubMed] [Google Scholar]
- DICKERSON J. W. The effect of growth on the composition of avian muscle. Biochem J. 1960 Apr;75:33–37. doi: 10.1042/bj0750033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B. Is acetylcholine the trophic neuromuscular transmitter? Arch Neurol. 1967 Aug;17(2):206–218. doi: 10.1001/archneur.1967.00470260096011. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Filogamo G., Gabella G. The development of neuro-muscular correlations, in vertebrates. Arch Biol (Liege) 1967;78(1):9–60. [PubMed] [Google Scholar]
- Fonnum F. Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J. 1969 Nov;115(3):465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN B. C., SIZER I. W. EFFECTS OF SPINAL CORD AND SUBSTRATE ON ACETYLCHOLINESTERASE IN CHICK EMBRYONIC SKELETAL MUSCLE. Dev Biol. 1965 Feb;11:136–153. doi: 10.1016/0012-1606(65)90041-2. [DOI] [PubMed] [Google Scholar]
- Giacobini G. Embryonic and postnatal development of choline acetyltransferase activity in muscles and sciatic nerve of the chick. J Neurochem. 1972 May;19(5):1401–1403. doi: 10.1111/j.1471-4159.1972.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Giacobini G., Marchisio P. C., Giacobini E., Koslow S. H. Developmental changes of cholinesterases and monoamine oxidase in chick embryo spinal and sympathetic ganglia. J Neurochem. 1970 Aug;17(8):1177–1185. doi: 10.1111/j.1471-4159.1970.tb03366.x. [DOI] [PubMed] [Google Scholar]
- HEBB C. O., KRNJEVIC K., SILVER A. ACETYLCHOLINE AND CHOLINE ACETYLTRANSFERASE IN THE DIAPHRAGM OF THE RAT. J Physiol. 1964 Jun;171:504–513. doi: 10.1113/jphysiol.1964.sp007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W. Release of neurotransmitters and their interaction with receptors. Annu Rev Biochem. 1972;41:925–952. doi: 10.1146/annurev.bi.41.070172.004425. [DOI] [PubMed] [Google Scholar]
- Israël M. Localisation de l'acétylcholine des synapses myoneurales et nerf-électroplaque. Arch Anat Microsc Morphol Exp. 1970 Apr-Jun;59(2):67–98. [PubMed] [Google Scholar]
- Karlsson E., Eaker D. L., Porath J. Purification of a neurotoxin from the venom of Naja nigricollis. Biochim Biophys Acta. 1966 Oct 31;127(2):505–520. doi: 10.1016/0304-4165(66)90404-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y., Chang C. C. Modes of actions of purified toxins from elapid venoms on neuromuscular transmission. Mem Inst Butantan. 1966;33(2):555–572. [PubMed] [Google Scholar]
- MCCAMAN R. E., HUNT J. M. MICRODETERMINATION OF CHOLINE ACETYLASE IN NERVOUS TISSUE. J Neurochem. 1965 Apr;12:253–259. doi: 10.1111/j.1471-4159.1965.tb06762.x. [DOI] [PubMed] [Google Scholar]
- MILLO A. [Comparative study of the cholinesterase content of striated muscle, heart and smooth muscle during the development of the chick embryo]. Riv Biol. 1961 Apr-Jun;54:251–261. [PubMed] [Google Scholar]
- Menez A., Morgat J. -L., Fromageot P., Ronseray A. -M., Boquet P., Changeux J. -P. Tritium labelling of the alpha-neurotoxin of Naja nigricollis. FEBS Lett. 1971 Oct 1;17(2):333–335. doi: 10.1016/0014-5793(71)80180-1. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- Patrick J., Heinemann S. F., Lindstrom J., Schubert D., Steinbach J. H. Appearance of acetylcholine receptors during differentiation of a myogenic cell line. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2762–2766. doi: 10.1073/pnas.69.10.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley K. L., Provine R. R. Neural correlates of embryonic motility in the chick. Brain Res. 1972 Oct 13;45(1):127–134. doi: 10.1016/0006-8993(72)90220-x. [DOI] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. Comparison between the effect of neuronal activity and nerve growth factor on the enzymes involved in the synthesis of norepinephrine. Pharmacol Rev. 1972 Jun;24(2):255–267. [PubMed] [Google Scholar]
- Thoenen H., Mueller R. A., Axelrod J. Trans-synaptic induction of adrenal tyrosine hydroxylase. J Pharmacol Exp Ther. 1969 Oct;169(2):249–254. [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Menez A., Fromageot P., Boquet P., Changeux J. P. Effet des agents cholinergiques et des anesthésiques locaux sur la cinétique de liaison de la toxine tritiée de Naja nigricollis au récepteur cholinergique. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 6;274(10):1575–1578. [PubMed] [Google Scholar]